��2013?��������ģ���£�N
2H
4���ǵ������γɵ�һ�ֻ����������ˮ����ҵ���������ط������£�ͬʱ�õ�����Ʒʮˮ��̼���ƣ��乤���������£�
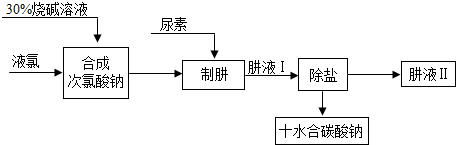
���¹��̵ķ�ӦΪ��CO��NH
2��
2�����أ�+NaClO+2NaOH=N
2H
4+Na
2CO
3+NaCl+H
2O��
��1��ʵ����������200g��������Ϊ30%������������Һ����Ҫˮ�����Ϊ
140
140
mL��ˮ���ܶȽ��ƿ���1g/cm
3��������ʱ������Ͳ��ȡ�����ˮ������װ���ѳ����õ��������ƹ����
�ձ�
�ձ�
��ò��������裬ʹ���ܽ⣬����ȴ�����£�����õ���Һװ���Լ�ƿ��������Ƥ����
���ñ�ǩ
���ñ�ǩ
���ŵ�ָ���ĵط���
��2����ʵ�����У�����Һ����������ȴ����ʮˮ��̼���ƾ�����֣�����ȥ�þ��壬�õ���Һ�ɲ��õIJ���Ϊ
D
D
��ѡ���ţ���
A������ B����ˮ����C���������� D������
��3��ʮˮ��̼���ƣ�Na
2CO
3?10H
2O��������ˮ��ˮ��Һ�ʼ��ԣ���pH��ֽ�ⶨ����Һ��pH�ķ�����
�ڰ״ɰ����Ƭ�Ϸ���һСƬpH��ֽ���ò�����պȡ̼������Һ���ε�pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�����pHֵ
�ڰ״ɰ����Ƭ�Ϸ���һСƬpH��ֽ���ò�����պȡ̼������Һ���ε�pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�����pHֵ
��
��4���º�������������Ӧ���ҷ�Ӧ��������Ⱦ���ʿ����ڳ�ȥ��¯���豸��ˮ���ܽ�������ȣ���д����������ʱ������Ӧ�Ļ�ѧ����ʽ��
N2H4+O2�TN2+2H2O
N2H4+O2�TN2+2H2O
��




 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�