
���� ������̼���������Ʒ�Ӧ������̼��Ƴ�����ˮ�����ݳ������������Լ��������̼�����������ݶ�����̼���������Լ���̼��þ�����������ɵ�����þ����������һ�����Լ���̼��þ��Ʒ��̼��þ������������
��� �⣺��1���跴Ӧ���ɶ�����̼������Ϊx��
Ca��OH��2+CO2�TCaCO3��+H2O��
44 100
x 5g
$\frac{44}{x}=\frac{100}{5g}$
x=2.2g
����������þ������Ϊx��̼��þ������Ϊy��
MgCO3+H2SO4=MgSO4+H2O+CO2����
84 120 44
y x 2.2g
$\frac{84}{y}=\frac{120}{x}=\frac{44}{2.2g}$
x=6g
y=4.3g
��2�����õ���ɫ����Һ�У����ʵ���������Ϊ$\frac{6g+��6g-4.3g��}{6g+50g-2.2g}��$��14.3%��
��3����Ʒ��̼��þ����������=$\frac{4.3g}{6g}��$100%��71.7%��80%�����ϸ�
�𰸣�
��1��2.2��
��2�����õ���ɫ����Һ�У����ʵ���������Ϊ14.3%��
��3�����ϸ�
���� ������Ҫ����ѧ�����û�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ȼ�ƾ��� | B�� |  ϡ��Ũ���� | C�� |  ���������� | D�� |  ��������ζ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ֻ�����һ��ʵ�鷽�� | |
| B�� | �Ȼ�ͭ��Һ�Dz��ɻ�ȱ���Լ� | |
| C�� | ѡ�����ֺ��ʵ��Լ�����ʵ��ʵ��Ŀ�� | |
| D�� | �Ȼ�����Һ�м���ͭ����̽���������ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 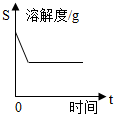 �͵����������м��������� | |
| B�� | 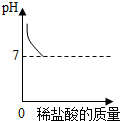 ������������Һ�еμ�ϡ������ǡ����ȫ��Ӧ | |
| C�� |  ��������������ͭ��Һ����μ�������������Һ | |
| D�� |  ��������������þ������п���������������������������Һ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
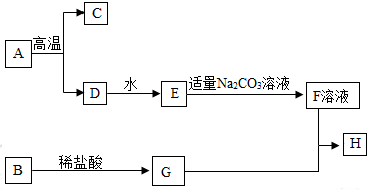 ���п�ͼ�е����ʾ�Ϊ��ѧ��ѧ�������ʣ�����A�Ǵ���ʯ����Ҫ�ɷ֣�B���������Ҫ�ɷ֣�H�Ǻ��ɫ��������ͼ������֮���ת����ϵ����ش�
���п�ͼ�е����ʾ�Ϊ��ѧ��ѧ�������ʣ�����A�Ǵ���ʯ����Ҫ�ɷ֣�B���������Ҫ�ɷ֣�H�Ǻ��ɫ��������ͼ������֮���ת����ϵ����ش��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com