
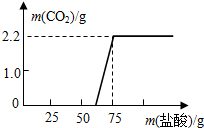
| Na2CO3������/g | ________ |
| ����NaOH������/g | ________ |
| NaOH�ı��ʳ̶� | ________ |
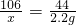
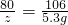
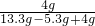 ��100%=33.3%��
��100%=33.3%��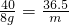
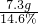 =50g��
=50g��| Na2CO3������/g | 5.3 |
| ����NaOH������/g | 4.0 |
| NaOH�ı��ʳ̶ȣ�������������ʾ�� | 33.3% |


 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 34��ij�о���ѧϰС��̽��CuSO4��Һ��NaOH��Һ�ķ�Ӧ���
34��ij�о���ѧϰС��̽��CuSO4��Һ��NaOH��Һ�ķ�Ӧ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
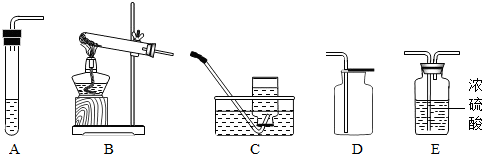
| ��ȡ���� | ����ҪƷ | װ������˳�� | ��Ӧ�Ļ�ѧ����ʽ |
| ���� | ����غͶ������� | ||
| ������̼����� | ����ʯ��ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| Na2CO3������/g | 5.3 5.3 |
| ����NaOH������/g | 4 4 |
| NaOH�ı��ʳ̶� | 33.3% 33.3% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС���ͬѧ����������װ��̽����ɫֲ������������Ƿ���
CO2�����������Ƶ�̽���������£���ش����е��й����⣮

��1�����裺��ɫֲ���ں�����������CO2���������
��2����Ʒ�����ʹ��ɫֲ���ڱܹ�ĺڰ��������������ã�������������в��������壮
��3���������ϣ�����ɫֲ�������ù��̣�ˮ+������̼һ���л���+����
����ɫֲ��������ù��̣��л���+����һ��������̼+ˮ+����
��4��ʵ�飺
| �������� | ��� |
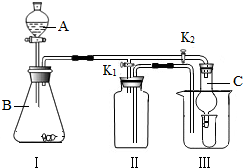
| �� ����װ�ð���ͼ ��ʾ���Ӻò�װ�뻯ѧ�Լ��� C�з�����ɫֲ�� | �� Aװ�õ������� �� Bװ�õ������� �� C�������ֲ������ԭ����________________
|
| �� ��A�ĵ��ܿڻ������� �������һ��ʱ�� | �� Aװ����Ӧ�۲쵽��������ʯ��ˮ����� ��Dװ����Ӧ�۲쵽��������ʯ��ˮ����� |
��5�����������ۣ����ж����̽��ʵ��________����ɹ������ɹ�������
��������о���ѧϰС��̽��ʵ�鲻�ɹ���ԭ���Ƕ��ġ������ʵ�������������ҳ����ܵ�һ��ԭ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС���ͬѧ����������װ��̽����ɫֲ������������Ƿ���
CO2�����������Ƶ�̽���������£���ش����е��й����⣮

��1�����裺��ɫֲ���ں�����������CO2���������
��2����Ʒ�����ʹ��ɫֲ���ڱܹ�ĺڰ��������������ã�������������в��������壮
��3���������ϣ�����ɫֲ�������ù��̣�ˮ+������̼һ���л���+����
����ɫֲ��������ù��̣��л���+����һ��������̼+ˮ+����
��4��ʵ�飺
| �������� | ��� |
| �� ����װ�ð���ͼ ��ʾ���Ӻò�װ�뻯ѧ�Լ���C�з�����ɫֲ�� | �� Aװ�õ������� �� Bװ�õ������� �� C�������ֲ������ԭ����________________
|
| �� ��A�ĵ��ܿڻ������� �������һ��ʱ�� | �� Aװ����Ӧ�۲쵽��������ʯ��ˮ����� �� Dװ����Ӧ�۲쵽��������ʯ��ˮ����� |
��5�����������ۣ����ж����̽��ʵ��________����ɹ������ɹ�������
��������о���ѧϰС��̽��ʵ�鲻�ɹ���ԭ���Ƕ��ġ������ʵ�������������ҳ����ܵ�һ��ԭ��________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com