
���� ��1�����ݼ״�ȼ�����ɶ�����̼��ˮ�������غ㶨�ɷ������
��2���������ʵļ��鷽�����з������
��3�����ݻ�ѧʽ�IJ��ȷ�����ʵ����
��� �⣺��1���״�ȼ�����ɶ�����̼��ˮ����ȼ�յĻ�ѧ����ʽΪ��2CH3OH+3O2$\frac{\underline{\;��ȼ\;}}{\;}$2CO2+4H2O��
��2���״�ȼ�����ɶ�����̼����ʹ�ó����ʯ��ˮ���飬�������ʯ��ˮ��
��3���ڼ״����Ҵ��У�̼��Ԫ�ص������Ȳ�ͬ�����������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ�ص�������������䣬�ʿ���ͨ���Ӳⶨ�Ķ�����̼��ˮ�������������̼��Ԫ�ص������ȣ��Ӷ����״����Ҵ��ֿ�����̼��Ԫ�ص�������Ϊ3��1����Ϊ�״�����̼��Ԫ�ص�������Ϊ4��1����Ϊ�Ҵ����ʴ�Ϊ�����������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ�ص�������������䣬�ʿ���ͨ���Ӳⶨ�Ķ�����̼��ˮ�������������̼��Ԫ�ص������ȣ��Ӷ����״����Ҵ��ֿ�����̼��Ԫ�ص�������Ϊ3��1����Ϊ�״�����̼��Ԫ�ص�������Ϊ4��1����Ϊ�Ҵ���
���� ���⿼����dz����Ŀ�ȼ������ʣ���ɴ��⣬�����������е����ʵ����ʽ��У�


 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ʿ��ɷ��ӡ�ԭ�ӻ����ӹ��� | |
| B�� | �ṹʾ��ͼΪ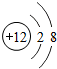 �� ��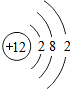 �����ӣ�����ͬ��Ԫ�� �����ӣ�����ͬ��Ԫ�� | |
| C�� | ���ԭ������Ϊm��ԭ��M��������n�����ӣ������������Ϊ��m-n | |
| D�� | ������̼����������Ԫ�غ�һ��̼Ԫ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | O2 | B�� | SO2 | C�� | H2CO3 | D�� | KClO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Ŀ�� | ���� | |
| A | ������������� | ����ζ |
| B | ����ͭ���ߺ������� | �۲쵼����ɫ |
| C | ����ˮ�ͳ���ʯ��ˮ | ͨ�������̼ |
| D | ���������Ͷ�����̼ | ����ȼ�ŵ�Сľ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ���� | C�� | ���� | D�� | ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com