
���� ����A��B��C�dz��л�ѧ�������ʣ���ˮ��Һ��Ϊ��ɫ��ȡ����A����Һ���Թ��У������м���������ɫ��ĩ����Һð���������ݣ����������������ȼ�ԣ����Ը�����������������A�ǹ���������Һ�����������ڶ������̵Ĵ�����������ˮ��������B��һ�ֳ������ᣬȡ����B����Һ���Թ��У������м���������ɫ���壬������ɫ����ζ����ʹ����ʯ��ˮ����ǵ����壬���Ը������Ƕ�����̼����ɫ������һ�����е�Ԫ����̼����Ԫ�أ�C��С�մ�����C��̼�����ƣ�ȡ����C����Һ���Թ��У��������ʯ��ˮ���а�ɫ����������̼�����ƺ��������Ʒ�Ӧ����̼��Ƴ������������ƺ�ˮ�����˺�����Һ�м���ϡ���ᣬ����������˵��̼�����Ʋ��������з�����
��� �⣺A��B��C�dz��л�ѧ�������ʣ���ˮ��Һ��Ϊ��ɫ��
��1��ȡ����A����Һ���Թ��У������м���������ɫ��ĩ����Һð���������ݣ����������������ȼ�ԣ����Ը�����������������A�ǹ���������Һ�����������ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��2��B��һ�ֳ������ᣬȡ����B����Һ���Թ��У������м���������ɫ���壬������ɫ����ζ����ʹ����ʯ��ˮ����ǵ����壬���ɫ������һ�����е�Ԫ����̼����Ӧ�����Һ�м���BaCl2��Һ��������ɫ��������B��ϡ���ᣮ������ɫ�����Ļ�ѧ����ʽΪBaCl2+H2SO4=BaSO4��+2HCl��
��3��C��С�մ�����C��̼�����ƣ�ȡ����C����Һ���Թ��У��������ʯ��ˮ���а�ɫ����������̼�����ƺ��������Ʒ�Ӧ����̼��Ƴ������������ƺ�ˮ�����˺�����Һ�м���ϡ���ᣬ����������˵��̼�����Ʋ�����������
����Һ�е����ʿ�����NaOH��
�ڼ������һ�������ķ�Ӧ���������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ�����䷴Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl�TNaCl+H2O��
��ͨ���Ƶ���֪�����˺�����Һ�м���ϡ���ᣬ����������˵������̼�����ƣ����ܺ����������ƣ��Լ����ɵ��������ƣ�����
A��ȡ������Һ���Թ��У��μӷ�̪���۲���Һ�Ƿ��죬�������ơ��������ƶ���ʹ��̪��죬��A����
B���������ƻ��������̼����̼��Ƴ�������B��ȷ��
C���������ƺ�̼���ƻ�����̼��Ƴ������������ƣ���C��ȷ��
��ѡ��BC��
�ʴ�Ϊ��
��1��˫��ˮ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��2��C��H2SO4��BaCl2+H2SO4=BaSO4��+2HCl��
��3����NaOH��Ca��OH��2��NaOH����NaOH+HCl=NaCl+H2O����B��C��
���� �ڽ������ʱ�����Ƚ������������������Ƴ���Ȼ�����Ƴ������ʺ����е�ת����ϵ�Ƶ�ʣ������ʣ�����Ƴ��ĸ������ʴ���ת����ϵ�н�����֤���ɣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ҺN��һ������Zn2+ | B�� | ��ҺM����ɫ����ɫ | ||
| C�� | ����N��һ������Zn��Fe��Cu | D�� | ����N������С�ڼ���п�۵����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
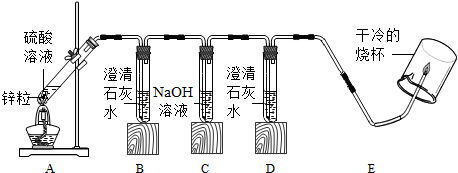
| A������� �������� | ʵ������ | |||
| B�� | D�� | E�� | ||
| ʵ��һ | 98% | ����� | ������� | ����ȼ |
| ʵ��� | 50% | �Ա���� | ������� | ��ȼ |
| ʵ���� | 25% | ������� | ������� | ��ȼ |
 ������д���������ڡ�SO2������д��ѧʽ����
������д���������ڡ�SO2������д��ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
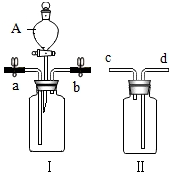 ������ͼװ�ý����й��������ʵ�ʵ�飮
������ͼװ�ý����й��������ʵ�ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | N2 | B�� | NO2 | C�� | HNO3 | D�� | NH3•H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ�� | B�� | ����������� | C�� | �������¶� | D�� | ����ˮ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com