�⣺��1�����⼣�߰ߵ��������뵽�����У��������������ᷴӦ�����ⷴӦ����������ᷴӦ�����ԣ���Ӧ�������ǣ����ȿ����⼣�ܽ⣬��Һ�ʻ�ɫ����һ����ֻῴ��������ð������Һ�ɻ�ɫ��Ϊ����ɫ��Һ��
��2����������Ӧ��֪����Һ�к���FeCl
3��FeCl
2�����������ǹ����ģ�����ʣ���HCl��
��3�������������ᷴӦ������������ͬʱ�ų������������ԣ��μӷ�Ӧ�����뷴Ӧǰ��Һ������֮�͵��ڷ�Ӧ����Һ�����������ɵ�����������֮�ͣ�
��4�����ɵ�����������Ϊ��7.0g+100g-106.8g=0.2g
����������Ϊx
Fe+2HCl=FeCl
2+H
2��
56 2
x 0.2g

��ã�x=5.6g
�����������������������Ϊ
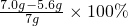
=20%
�ʴ�Ϊ����1�����ȿ����⼣�ܽ⣬��Һ�ʻ�ɫ����һ����ֻῴ��������ð������Һ�ɻ�ɫ��Ϊ����ɫ��Һ��2��FeCl
3��FeCl
2��HCl����3���������ᷴӦ�����������������������μӷ�Ӧ�����뷴Ӧǰ��Һ������֮�͵��ڷ�Ӧ����Һ�����������ɵ�����������֮�ͣ���4�������������������������Ϊ20%��
��������1�����⼣�߰ߵ��������뵽�����У��������������ᷴӦ�����ⷴӦ����������ᷴӦ���ݴ˻ش�Ӧ������
��2������������Ӧ������Һ�е����ʣ�
��3�������������ᷴӦ�ų�����������Һ�����ı仯��
��4�������������ᷴӦ�������غ㶨��������ɵ���������������������������������������������������������������������������������������
���������⿼�������������Լ��������������֮��ķ�Ӧ���������������������鵽�����ݣ���ɴ�����Ŀ��������������֪ʶ�����ش���Ҫͬѧ����ƽ����ѧϰ�м�ǿ����֪ʶ�Ĵ������Ա����Ӧ�ã�

 ��ã�x=5.6g
��ã�x=5.6g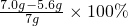 =20%
=20%

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�