
 =60%£¬Čē¹ūijĶ¬Ń§ŌŚ¶ĮČ”Ķ¼ÖŠĮæĶ²Źż¾ŻŹ±ø©ŹÓ¶ĮŹż£¬ŅņĪŖĻÖŌŚø©ŹÓ¶ĮŹżµÄŹĒµ¹Į¢µÄĮæĶ²£¬ĖłŅŌ¶ĮČ”µÄĘųĢåĢå»ż»įĘ«Š”£»¹ŹĢī£ŗCO2+2NaOHØTNa2CO3+H2O£»³ĪĒåŹÆ»ŅĖ®²»±ä»ė×Ē£»60%£»£¼£»
=60%£¬Čē¹ūijĶ¬Ń§ŌŚ¶ĮČ”Ķ¼ÖŠĮæĶ²Źż¾ŻŹ±ø©ŹÓ¶ĮŹż£¬ŅņĪŖĻÖŌŚø©ŹÓ¶ĮŹżµÄŹĒµ¹Į¢µÄĮæĶ²£¬ĖłŅŌ¶ĮČ”µÄĘųĢåĢå»ż»įĘ«Š”£»¹ŹĢī£ŗCO2+2NaOHØTNa2CO3+H2O£»³ĪĒåŹÆ»ŅĖ®²»±ä»ė×Ē£»60%£»£¼£»


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
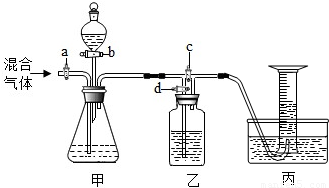
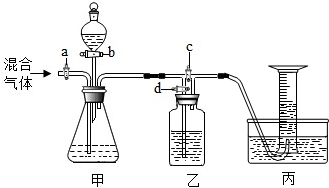
 £Ø2012?·æɽĒųŅ»Ä££©ÓĆČēĶ¼µÄ×°ÖĆ½«Ņ»¶ØĮæµÄCO2ŗĶCOµÄ»ģŗĻĘųĢå½ųŠŠ·ÖĄėŗĶøÉŌļ£®Ķ¼ÖŠµÄa”¢b”¢c”¢d¾łĪŖ»īČū£¬æÉŅŌæŲÖĘĘųĢåµÄĶعżŗĶŅŗĢåµÄ¼ÓČė£¬ŹµŃéĒ°»īČū¾łŅŃ¹Ų±Õ£®ĒėŃ”ŌńŹŹŅĖµÄŹŌ¼ĮĶź³ÉÉĻŹöŹµŃ飮¹©Ń”ÓƵďŌ¼ĮÓŠ£ŗ¢ŁĻ”ĮņĖį¢ŚÅØĮņĖį¢ŪĒāŃõ»ÆÄĘČÜŅŗ¢Ü³ĪĒåµÄŹÆ»ŅĖ®£ØŹŌ¼Į¾ł×ćĮ棩
£Ø2012?·æɽĒųŅ»Ä££©ÓĆČēĶ¼µÄ×°ÖĆ½«Ņ»¶ØĮæµÄCO2ŗĶCOµÄ»ģŗĻĘųĢå½ųŠŠ·ÖĄėŗĶøÉŌļ£®Ķ¼ÖŠµÄa”¢b”¢c”¢d¾łĪŖ»īČū£¬æÉŅŌæŲÖĘĘųĢåµÄĶعżŗĶŅŗĢåµÄ¼ÓČė£¬ŹµŃéĒ°»īČū¾łŅŃ¹Ų±Õ£®ĒėŃ”ŌńŹŹŅĖµÄŹŌ¼ĮĶź³ÉÉĻŹöŹµŃ飮¹©Ń”ÓƵďŌ¼ĮÓŠ£ŗ¢ŁĻ”ĮņĖį¢ŚÅØĮņĖį¢ŪĒāŃõ»ÆÄĘČÜŅŗ¢Ü³ĪĒåµÄŹÆ»ŅĖ®£ØŹŌ¼Į¾ł×ćĮ棩²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğĮÉÄžŹ”°°É½ŹŠÖŠæ¼Ņ»Ä£»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ÓĆČēĶ¼µÄ×°ÖĆ·ÖĄėCO2ŗĶCOµÄ»ģŗĻĘųĢå²¢²ā¶ØĘäÖŠCO2µÄĢå»ż·ÖŹż£®Ķ¼ÖŠa£¬b£¬c£¬d¾łĪŖ»īČū£¬æÉŅŌæŲÖĘĘųĢåµÄĶعżŗĶŅŗĢåµÄ¼ÓČė£®ĒėŃ”ŌńŹŹŅĖµÄŹŌ¼ĮĶź³ÉŹµŃé
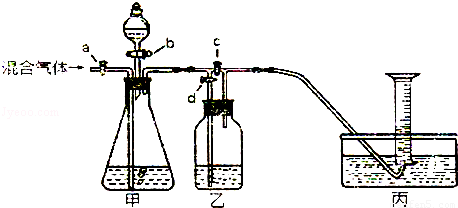
¹©Ń”ÓƵďŌ¼ĮÓŠ£ŗ¢ŁĻ”ĮņĖį£»¢Ś³ĪĒåµÄŹÆ»ŅĖ®£»¢ŪĒāŃõ»ÆÄĘČÜŅŗ£ØŹŌ¼Į¾ł×ćĮ棩
ŹµŃé·ÖŅŌĻĀĮ½²½½ųŠŠ£ŗ
£Ø1£©¹Ų±Õb£¬c£¬“ņæŖa£¬dĶØČė»ģŗĻĘųĢ壮Ōņ¼×ÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ”” ””£®ČōŅŅ×°ÖĆÖŠµÄĻÖĻóŹĒ”” ””£¬ĖµĆ÷¼××°ÖĆÖŠ·“Ó¦ŹĒ³ä·ÖµÄ£®£Ø×¢£ŗ¼ŁÉč×°ÖĆÖŠµÄøĆĘųĢåČ«²æ±»»ŲŹÕ£©±ū×°ÖĆÖŠÓĆ³äĀśĖ®µÄµ¹Į¢ĮæĶ²ŹÕ¼ÆĘųĢ壬ČōĶØČė500mL»ģŗĻĘųĢ壬ĮæĶ²ÖŠ¾Ķ»įŹÕ¼Æµ½200mLĘųĢ壬Ōņ»ģŗĻĘųĢåÖŠCO2µÄĢå»ż·ÖŹżĪŖ”” ””£»Čē¹ūijĶ¬Ń§ŌŚ¶ĮČ”Ķ¼ÖŠĮæĶ²Źż¾ŻŹ±ø©ŹÓ¶ĮŹż£¬ŌņĖł¶ĮČ”µÄĘųĢåĢå»ż»į”” ””200mL£ØĢī”°£¾”±”°£¼”±»ņ”°=”±£©
£Ø2£©±Õ¹Ųa£¬d£¬“ņæŖb£¬c£¬ČĆ·ÖŅŗĀ©¶·ÖŠŹŌ¼Į»ŗ»ŗĮ÷ĻĀ¼“æɲśÉśĮķŅ»ÖÖĘųĢ壮ŌņŹÕ¼ÆøĆĘųĢåÓ¦øĆ
Ź¹ÓĆ”””” ·Ø
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012Äź±±¾©ŹŠ·æɽĒųÖŠæ¼»ÆѧŅ»Ä£ŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
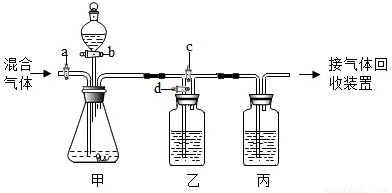 ÓĆČēĶ¼µÄ×°ÖĆ½«Ņ»¶ØĮæµÄCO2ŗĶCOµÄ»ģŗĻĘųĢå½ųŠŠ·ÖĄėŗĶøÉŌļ£®Ķ¼ÖŠµÄa”¢b”¢c”¢d¾łĪŖ»īČū£¬æÉŅŌæŲÖĘĘųĢåµÄĶعżŗĶŅŗĢåµÄ¼ÓČė£¬ŹµŃéĒ°»īČū¾łŅŃ¹Ų±Õ£®ĒėŃ”ŌńŹŹŅĖµÄŹŌ¼ĮĶź³ÉÉĻŹöŹµŃ飮¹©Ń”ÓƵďŌ¼ĮÓŠ£ŗ¢ŁĻ”ĮņĖį¢ŚÅØĮņĖį¢ŪĒāŃõ»ÆÄĘČÜŅŗ¢Ü³ĪĒåµÄŹÆ»ŅĖ®£ØŹŌ¼Į¾ł×ćĮ棩
ÓĆČēĶ¼µÄ×°ÖĆ½«Ņ»¶ØĮæµÄCO2ŗĶCOµÄ»ģŗĻĘųĢå½ųŠŠ·ÖĄėŗĶøÉŌļ£®Ķ¼ÖŠµÄa”¢b”¢c”¢d¾łĪŖ»īČū£¬æÉŅŌæŲÖĘĘųĢåµÄĶعżŗĶŅŗĢåµÄ¼ÓČė£¬ŹµŃéĒ°»īČū¾łŅŃ¹Ų±Õ£®ĒėŃ”ŌńŹŹŅĖµÄŹŌ¼ĮĶź³ÉÉĻŹöŹµŃ飮¹©Ń”ÓƵďŌ¼ĮÓŠ£ŗ¢ŁĻ”ĮņĖį¢ŚÅØĮņĖį¢ŪĒāŃõ»ÆÄĘČÜŅŗ¢Ü³ĪĒåµÄŹÆ»ŅĖ®£ØŹŌ¼Į¾ł×ćĮ棩²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com