
���� ��1������ú�к�����Ԫ�أ�ȼ�������ɶ���������
��2�����ݼ���ȼ�����ɶ�����̼��ˮ���з�������������Դ�벻��������Դ�ĸ���Ϳ�ȼ���������������
��� �⣺
��1��ú�к�����Ԫ�أ�ȼ�������ɶ���������Ⱦ��������ѧʽΪ��SO2��
��2������ȼ�������˶�����̼��ˮ����Ӧ�ķ���ʽ�ǣ�CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O����ȼ������һ������Դ�����ڲ���������Դ��
�𰸣�
��1��SO2��
��2��CH4+2O2$\frac{\underline{\;��ȼ\;}}{\;}$CO2+2H2O������������
���� ������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
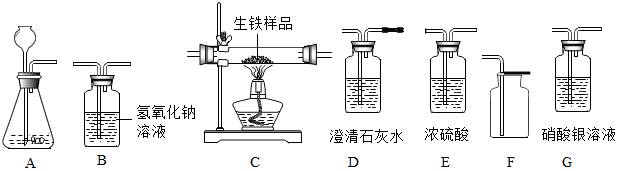
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
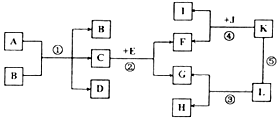 A-L�dz��л�ѧ���������ʣ����ǵ��ת����ϵ��ͼ��ʾ�����ַ�Ӧ��������ȥ������������ʾ���ʼ����ת����ϵ����-����ʾ���������ܷ�����Ӧ�����Т��dz�����һ����Ҫ�ĵ�ζƷ�������ڷ�����L�Ǵ���ʯ����Ҫ�ɷ֣�
A-L�dz��л�ѧ���������ʣ����ǵ��ת����ϵ��ͼ��ʾ�����ַ�Ӧ��������ȥ������������ʾ���ʼ����ת����ϵ����-����ʾ���������ܷ�����Ӧ�����Т��dz�����һ����Ҫ�ĵ�ζƷ�������ڷ�����L�Ǵ���ʯ����Ҫ�ɷ֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
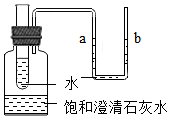 ��ͼ��ʾ�����ƿ��ʢ���������ͳ���ʯ��ˮ��С�Թܺ�U�ι��о�������ˮ������С�Թ���ע������Ũ���ᣬ��ش�
��ͼ��ʾ�����ƿ��ʢ���������ͳ���ʯ��ˮ��С�Թܺ�U�ι��о�������ˮ������С�Թ���ע������Ũ���ᣬ��ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� |  �������� �������� | B�� |  ��ʯ�Ҹ����������� ��ʯ�Ҹ����������� | ||
| C�� |  С�մ������ͷ� | D�� |  �ѵ���������� �ѵ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������Դ | |
| B�� | �ÿɽ�������������װ�л��ͺ� | |
| C�� | ����ʹ��ũҩ��������������ʳ���� | |
| D�� | ����������ʹԭ�Ͼ����ܵ�ת��Ϊ��Ʒ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
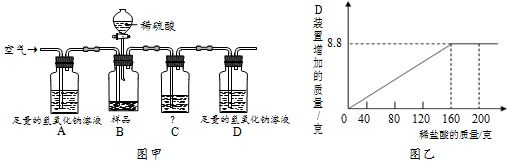
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com