
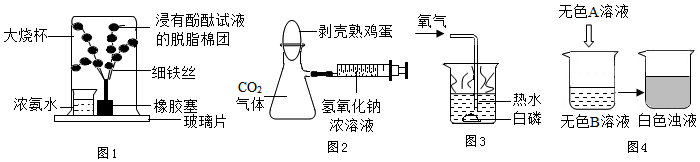
·ÖĪö £Ø1£©øł¾ŻĻ“½ą¾«¾ßÓŠČé»Æ×÷ÓĆ·ÖĪö£®
£Ø2£©øł¾ŻĪ¬ÉśĖŲµÄÉśĄķ×÷ÓĆ·ÖĪö»Ų“š£»
£Ø3£©øł¾Ż»ÆѧŌŖĖŲÓėČĖĢ彔浵ĹŲĻµĄ“·ÖĪö£»
£Ø4£©øł¾ŻÓ²Ė®Čķ»ÆµÄ·½·Ø½ā“š£»
£Ø5£©øł¾ŻĆš»šŌĄķ½ā“š£»
£Ø6£©øł¾ŻµĶĢ¼Éś»īµÄ×ö·Ø½ā“š£»
£Ø7£©øł¾Ż·ĄÖ¹ĢśÉśŠāµÄ·½·Ø½ā“š£®
½ā“š ½ā£ŗ£Ø1£©ÓĆĻ“½ą¾«ĒåĻ“ÓĶĪŪ£¬ŹĒĄūÓĆĮĖĻ“½ą¾«µÄČé»Æ×÷ÓĆ£»¹ŹĢī£ŗČé»Æ£»
£Ø2£©“óŌęŗ¬ÓŠµ°°×ÖŹ”¢ĢĒĄą¼°Ī¬ÉśĖŲµČÓŖŃųĖŲ£¬ĘäÖŠĘšµ½µ÷½ŚČĖĢåŠĀ³Ā“śŠ»”¢Ō¤·Ą¼²²”×÷ÓƵÄÓŖŃųĖŲŹĒĪ¬ÉśĖŲ£»¹ŹĢī£ŗĪ¬ÉśĖŲ£»
£Ø3£©ĒąÉŁÄźČ±øĘŅ×»¼ŲžŁĶ²”£»¹ŹĢī£ŗøĘ£»
£Ø4£©Ó²Ė®±äČķĖ®Éś»īÖŠæÉÓĆÖó·Š·½·Ø£®
£Ø5£©³“²ĖŹ±ÓĶ¹ųÖŠµÄÓĶ²»É÷×Å»š£¬æÉÓĆ¹ųøĒøĒĆš£¬ĘäĆš»šŌĄķĪŖøō¾ųŃõĘų£»
£Ø6£©Ģį³«”°µĶĢ¼Éś»ī”±£¬³öŠŠ¾”Įæ²½ŠŠ”¢³Ė×ų¹«½»³µ”¢²»ÓĆŅ»“ĪŠŌæź×Ó£¬½ŚŌ¼Ö½ÕÅµČ£®
£Ø7£©×ŌŠŠ³µĮ“ĢõµÄ·ĄŠā“ėŹ©ĶæÓĶ£®
“š°ø£ŗ£Ø1£©Čé»Æ£»£Ø2£©Ī¬ÉśĖŲ£»£Ø3£©øĘ£»£Ø4£©Öó·Š£»£Ø5£©øō¾ųŃõĘų£»£Ø6£©³Ė×ų¹«½»³µ£ŗ£Ø7£©ĶæÓĶ£®
µćĘĄ ±¾Ģāæ¼²éĮĖÓėÉś»īĮŖĻµĆÜĒŠµÄ»ÆѧÖŖŹ¶£¬ČĆѧɜĢå»į»ÆѧĄ“Ō“ÓŚÉś»ī·žĪńÓŚÉś»ī£®»ÆѧÓėĪŅĆĒµÄÉś»īĻ¢Ļ¢Ļą¹Ų£¬ÓėÉś²ś”¢Éś»īĻą¹ŲµÄÖŖŹ¶ŹĒÖŠæ¼æ¼²éµÄČȵćÖ®Ņ»£¬Įé»īŌĖÓĆĖłŃ§ÖŖŹ¶ŹĒÕżČ·½ā“š±¾ĢāµÄ¹Ų¼ü£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 JD PizzaŹĒ³Ō»õĆĒĻ²»¶µÄæģ²ĶĆĄŹ³Ö®Ņ»£¬ĖüÖÖĄą·±¶ąÓÖÓŖŃų·įø»£¬ĘäÖŠµÄµŲ¹ĻÅūČųøüŹĒŹÜµ½ČĖĆĒµÄĒąķł£®øĆÅūČųŹĒŌŚĆ걿ÉĻ¼ÓČėµŲ¹Ļ”¢Ēą½·µČŌĮĻŗęæ¾ÖĘµĆ£®Ēė»Ų“šĻĀĮŠĪŹĢā
JD PizzaŹĒ³Ō»õĆĒĻ²»¶µÄæģ²ĶĆĄŹ³Ö®Ņ»£¬ĖüÖÖĄą·±¶ąÓÖÓŖŃų·įø»£¬ĘäÖŠµÄµŲ¹ĻÅūČųøüŹĒŹÜµ½ČĖĆĒµÄĒąķł£®øĆÅūČųŹĒŌŚĆ걿ÉĻ¼ÓČėµŲ¹Ļ”¢Ēą½·µČŌĮĻŗęæ¾ÖĘµĆ£®Ēė»Ų“šĻĀĮŠĪŹĢā²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | 2H2O$\frac{\underline{\;Ķصē\;}}{\;}$2H2”ü+O2”ü | B£® | CO+CuO$\frac{\underline{\;¼ÓČČ\;}}{\;}$Cu+CO2 | ||
| C£® | CaO+H2OØTCa£ØOH£©2 | D£® | 2NaOH+H2SO4=Na2SO4+2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ½«ÉśĢś·ŪÄ©Ķ¶ČėŹ¢ÓŠ¹żĮæŃĪĖįµÄÉÕ±ÖŠ£¬³ä·Ö·“Ó¦ŗóČŌÓŠŗŚÉ«²ŠŌü | |
| B£® | ĮņŌŚæÕĘųÖŠČ¼ÉÕ·¢³öµĄ¶É«µÄ»šŃę | |
| C£® | ¼ŅĶ„Š”ŹµŃéÖŠ£¬½«Ź³“×Óė“æ¼ī»ģŗĻÓŠ“óĮæĘųÅŻ²śÉś | |
| D£® | ĢśĖæŌŚŃõĘųÖŠČ¼ÉÕ·¢³ö°×¹ā£¬Éś³ÉŗŚÉ«¹ĢĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ±ū°±ĖįŹōÓŚĪŽ»ś»ÆŗĻĪļ | |
| B£® | ±ū°±ĖįÖŠĢ¼”¢ĒāŌŖĖŲµÄÖŹĮæ±ČĪŖ3£ŗ1 | |
| C£® | ±ū°±Ėį»ÆѧŹ½ÖŠx=7 | |
| D£® | Ćæøö±ū°±Ėį·Ö×ÓÖŠŗ¬ÓŠŅ»øöŃõĘų·Ö×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com