
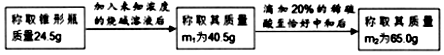
���� ��1��ϡ���������=65.0g-40.5g=24.5g��
��2�����ݻ�ѧ����ʽ���������������Լ�����������Ƶ�������������������ʵ�����������
��3������pHֵ��֪��������������ʼ�����Ľ��Ҫƫ��
��� �⣺��1��ϡ���������=65.0g-40.5g=24.5g�����������Ϊ24.5g��20%=4.9g��
��2�����ռ���Һ����������Ϊx��
2NaOH+H2SO4=Na2SO4+2H2O
80 98
x 4.9g
$\frac{80}{x}=\frac{98}{4.9g}$��
��ã�x=4.0g
��ƿ�ռ���Һ����������������$\frac{4.0g}{40.5g-24.5g}��100%$=25.0%��
��3������pH=6.2����֪��������������ʼ�����Ľ��Ҫƫ��
�𰸣���1��4.9����2��25.0%����3��ƫ�μӵ�ϡ���������
���� ������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ������������Ĺؼ��Ƿ�����Ӧ����������֮���������ϵ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̼��Ʒ� | B�� | ������þ�� | C�� | �����Ʒ� | D�� | ����þ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
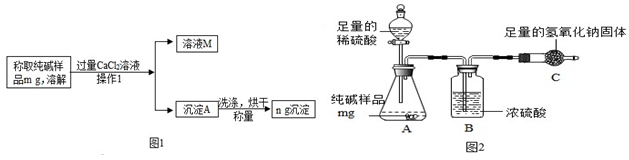
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������ | |
| B�� | �þ�����ϩ���������������ľ�Ե�� | |
| C�� | ����ʯ����ʳƷ����� | |
| D�� | ���¼Ʋ���ˮ��ָʾ�¶ȱ仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ڻ�����䣬�����������ܹ�ȼ�շų��������� | |
| B�� | ��̿�������������������ڽ�̿���������� | |
| C�� | ��ʯ�ҿ����ڸ���������������������ʯ���ܺ��ᷢ���кͷ�Ӧ | |
| D�� | ����Ʒ����Ϳ�����ۡ������ۣ������⣬���������Ļ�ѧ���ʱ����ȶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��ԭ�ӵĺ�����ӷ�5���Ų� | B�� | ��Ԫ�ص�ԭ������Ϊ38 | ||
| C�� | �����ӵĻ�ѧ����ΪSr2+ | D�� | �����ȵĻ�ѧ���ʲ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʯ����ˮ��Ӧ�ɼ���ʳ�� | |
| B�� | ��ѹ��������ÿ���������ѹǿ���е㽵�͵�ԭ�� | |
| C�� | ������ר�ÿ������л���̿�����������к����� | |
| D�� | ����Һ����Һ���ķе㲻ͬ���ɽ��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
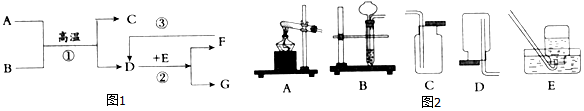
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com