
£Ø10ÉĒĶ·22£©(13·Ö)Ēėøł¾ŻĖłĢį¹©µÄŹŌ¼ĮŗĶŅĒĘ÷£¬°“ŅŖĒóĢīæÕ£ŗ
I£®ŹŌ¼Į£ŗĻ”ĮņĖį”¢Ļ”ŃĪĖį”¢¹żŃõ»ÆĒāČÜŅŗ”¢“óĄķŹÆ”¢¶žŃõ»ÆĆĢ
¢ņ£®ŅĒĘ÷£ŗ(Ģś¼ÜĢØ”¢ŹÆĆŽĶųµČŅŃŹ”ĀŌ)
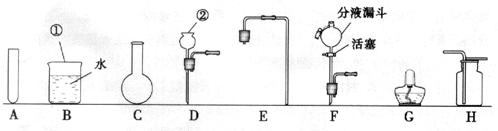
(1)Š“³öĶ¼ÖŠ±źÓŠ¢Ł”¢¢ŚŅĒĘ÷µÄĆū³Ę£ŗ¢Ł £¬¢Ś ”£
(2)ŹµŃéŹŅÖĘČ”ŃõĘųŹ±£¬ĪŖĮĖ±ćÓŚæŲÖĘ·“Ó¦ĒŅ»ńµĆĘ½ĪȵÄĘųĮ÷£¬·¢Éś×°ÖĆæÉŃ”ÓĆĶ¼ÖŠµÄ (Ģī×ÖÄø)£¬»Æѧ·½³ĢŹ½ĪŖ ”£ÓĆøĆ×°ÖĆŗĶĢį¹©µÄŹŌ¼Į»¹æÉŅŌÖĘČ” ĘųĢ壬»Æѧ·½³ĢŹ½ĪŖ ”£ŹÕ¼Æ·½·ØŹĒ £¬¼ģŃéøĆĘųĢåĀśĘæµÄ·½·ØŹĒ ”£
(3)Éč¼ĘŅ»Ģ×ÖĘȔɣĮæÕōĮóĖ®µÄ¼ņŅ××°ÖĆ£¬ŠčŃ”ŌńĶ¼ÖŠµÄA”¢BŗĶ (Ģī×ÖÄø)”£
 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
| 2 |
| 3 |
| ||
| ||
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ӣ
”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ğȫ¹śÖŠæ¼ÕęĢā·ÖĄą»ć±ą×ØĢāĖÄ ĘųĢåµÄÖʱø£Ø¶ž£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10ÉĒĶ·22£©(13·Ö)Ēėøł¾ŻĖłĢį¹©µÄŹŌ¼ĮŗĶŅĒĘ÷£¬°“ŅŖĒóĢīæÕ£ŗ
I£®ŹŌ¼Į£ŗĻ”ĮņĖį”¢Ļ”ŃĪĖį”¢¹żŃõ»ÆĒāČÜŅŗ”¢“óĄķŹÆ”¢¶žŃõ»ÆĆĢ
¢ņ£®ŅĒĘ÷£ŗ(Ģś¼ÜĢØ”¢ŹÆĆŽĶųµČŅŃŹ”ĀŌ)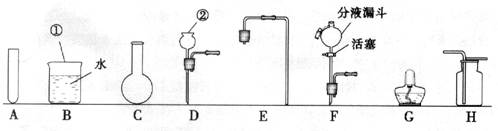
(1)Š“³öĶ¼ÖŠ±źÓŠ¢Ł”¢¢ŚŅĒĘ÷µÄĆū³Ę£ŗ¢Ł £¬¢Ś ”£
(2)ŹµŃéŹŅÖĘČ”ŃõĘųŹ±£¬ĪŖĮĖ±ćÓŚæŲÖĘ·“Ó¦ĒŅ»ńµĆĘ½ĪȵÄĘųĮ÷£¬·¢Éś×°ÖĆæÉŃ”ÓĆĶ¼ÖŠµÄ (Ģī×ÖÄø)£¬»Æѧ·½³ĢŹ½ĪŖ ”£ÓĆøĆ×°ÖĆŗĶĢį¹©µÄŹŌ¼Į»¹æÉŅŌÖĘČ” ĘųĢ壬»Æѧ·½³ĢŹ½ĪŖ ”£ŹÕ¼Æ·½·ØŹĒ £¬¼ģŃéøĆĘųĢåĀśĘæµÄ·½·ØŹĒ ”£
(3)Éč¼ĘŅ»Ģ×ÖĘȔɣĮæÕōĮóĖ®µÄ¼ņŅ××°ÖĆ£¬ŠčŃ”ŌńĶ¼ÖŠµÄA”¢BŗĶ (Ģī×ÖÄø)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2010ğȫ¹śÖŠæ¼ÕęĢā·ÖĄą»ć±ą×ØĢāĖÄĘųĢåµÄÖʱø£Ø¶ž£© ĢāŠĶ£ŗĢīæÕĢā
£Ø10ÉĒĶ·22£©(13·Ö)Ēėøł¾ŻĖłĢį¹©µÄŹŌ¼ĮŗĶŅĒĘ÷£¬°“ŅŖĒóĢīæÕ£ŗ
I£®ŹŌ¼Į£ŗĻ”ĮņĖį”¢Ļ”ŃĪĖį”¢¹żŃõ»ÆĒāČÜŅŗ”¢“óĄķŹÆ”¢¶žŃõ»ÆĆĢ
¢ņ£®ŅĒĘ÷£ŗ(Ģś¼ÜĢØ”¢ŹÆĆŽĶųµČŅŃŹ”ĀŌ)
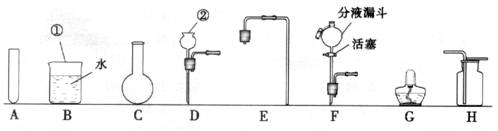
(1)Š“³öĶ¼ÖŠ±źÓŠ¢Ł”¢¢ŚŅĒĘ÷µÄĆū³Ę£ŗ¢Ł £¬¢Ś ”£
(2)ŹµŃéŹŅÖĘČ”ŃõĘųŹ±£¬ĪŖĮĖ±ćÓŚæŲÖĘ·“Ó¦ĒŅ»ńµĆĘ½ĪȵÄĘųĮ÷£¬·¢Éś×°ÖĆæÉŃ”ÓĆĶ¼ÖŠµÄ (Ģī×ÖÄø)£¬»Æѧ·½³ĢŹ½ĪŖ ”£ÓĆøĆ×°ÖĆŗĶĢį¹©µÄŹŌ¼Į»¹æÉŅŌÖĘČ” ĘųĢ壬»Æѧ·½³ĢŹ½ĪŖ ”£ŹÕ¼Æ·½·ØŹĒ £¬¼ģŃéøĆĘųĢåĀśĘæµÄ·½·ØŹĒ ”£
(3)Éč¼ĘŅ»Ģ×ÖĘȔɣĮæÕōĮóĖ®µÄ¼ņŅ××°ÖĆ£¬ŠčŃ”ŌńĶ¼ÖŠµÄA”¢BŗĶ (Ģī×ÖÄø)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com