
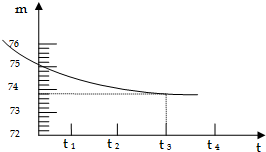
 ij��ɽ��ʯ��ʯ��Ʒ�к��ж����������ʣ�����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ��壩����ʦѧУ��ͬѧ����ⶨ����Ʒ��̼��Ƶ��������������Dz�ȡ��һ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����50g��ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ������������m���Ĺ�ϵ��ͼ��ʾ���Իش�
ij��ɽ��ʯ��ʯ��Ʒ�к��ж����������ʣ�����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ��壩����ʦѧУ��ͬѧ����ⶨ����Ʒ��̼��Ƶ��������������Dz�ȡ��һ��ʯ��ʯ��Ʒ����������Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����50g��ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ������������m���Ĺ�ϵ��ͼ��ʾ���Իش����� ��Ӧ��Ӵ�Խ��֣���Ӧ����Խ�죻
��Ӧǰ��������Ϊ��Ӧ���ɶ�����̼�����������ݶ�����̼���������Լ���̼��Ƶ���������һ�����Լ����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��� �⣺��1����ʯ��ʯ��Ʒ�������ҪĿ��������̼��ƺ�ϡ����ĽӴ�������ӿ췴Ӧ���ʣ�
����ӿ��ϡ���ᷴӦ�����ʣ�
��2��ʵ�����ʱ�����ų�������̼������Ϊ��6g+20g+50g-73.8g=2.2g��
���2.2��
��3����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2����
100 44
x 2.2g
$\frac{100}{x}$=$\frac{44}{2.2g}$��
x=5g��
��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ��$\frac{5g}{6g}$��100%=83.3%��
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ�����������83.3%��
���� �������ڼ����е�Ӧ�úܹ㷺�����Ĺؼ���Ҫ���������ʵ���������Ҫ���δ֪��֮��Ĺ�ϵ���ٸ��ݾ����������⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʢ��͵����Ϻм��з��� | |
| B�� | �س��Ž�ϴ�ȼ�ű��� | |
| C�� | �ü״��������������ȼ�� | |
| D�� | ����ũ������ոѷ��ճɲ�ľ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ɫ���� | B�� | ���ų����� | ||
| C�� | ����������Ӧ | D�� | �������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʯ���� | B�� | ���Ͻ� | C�� | ������ | D�� | ������Ʒ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuO | B�� | H2O | C�� | CO2 | D�� | HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ڹ�ũҵ�����о��й㷺��;��
������ڹ�ũҵ�����о��й㷺��;���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1����ͼ����ʾ����С�ձ�A��װ��30mL������ˮ���ڵμӼ��η�̪��Һ����Һ����ɫ����С�ձ�B��ʢ��Լ10mL��Ũ��ˮ���ô��ձ���סA��B��һ��ʱ���ɹ۲쵽�������ǣ��ձ�A��Һ����ɫ��죬��ʵ��˵�������Dz����˶��ģ�
��1����ͼ����ʾ����С�ձ�A��װ��30mL������ˮ���ڵμӼ��η�̪��Һ����Һ����ɫ����С�ձ�B��ʢ��Լ10mL��Ũ��ˮ���ô��ձ���סA��B��һ��ʱ���ɹ۲쵽�������ǣ��ձ�A��Һ����ɫ��죬��ʵ��˵�������Dz����˶��ģ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com