
��8�֣�(1)С���ӳ��й�����һЩʳƷ��������������Ϊ���ӳ������ڽ����˲�ͬ�Ĵ�����
�ٲ�����հ�װ����Ŀ���� ��
�ڲ��ó�����װ�����е���������� ��
�۷���һ����ʯ�ң���������(����ѧ����ʽ��ʾ) ��
�ܷ���һ����˫������--��ԭ�����ۣ������յ����������� ��
��2������H��C��O��Na���ֳ�����Ԫ�أ���ѡ�����е�Ԫ��д����������Ҫ������ʸ�һ�֣��û�ѧʽ��ʾ����
��ʳƷ�г��õ���ζ�� ���ڳ����е����Ե�ζ�� ��
����������¯�������ļ� ��������θ������һ���� ��
(1) �ٷ�ֹʳ����� �ڵ�����N2�� ��CaO + H2O = Ca(OH)2
��ˮ��������H2O �� O2��
��2����C12H22O11 ��CH3COOH ��NaOH ��NaHCO3
���������������1���ٲ�����հ�װ����Ŀ���ǣ�������������ֹʳ�����
�ڵ����Ļ�ѧ���ʲ����ã����Գ������������������Բ��ó�����װ�����е���������ǣ�������N2������ʯ������ˮ��Ӧ�����������������������������(�û�ѧ����ʽ��ʾ)��CaO + H2O = Ca(OH)2�ܷ���һ����˫������--��ԭ�����ۣ������������ǺͿ����е�ˮ�ֺ�������Ӧ�����������յ����������ǣ�ˮ��������H2O �� O2��
��2����ʳƷ�г��õ���ζ�������ǣ���ѧʽ��C12H22O11
�ڳ����е����Ե�ζ����������ʳ�λ�ʳ�ף�����û����Ԫ�أ�����ֻ����ʳ�ף���ѧʽ��CH3COOH����������¯�������ļNaOH
������θ������һ���Σ�NaHCO3
���㣺�������ʵ���������;



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(5��)�����Ļ�ѧ���ʱȽϻ��ã���֧��ȼ�ա���ͼ��ľ̿��������ȼ��ʵ��ʾ��ͼ��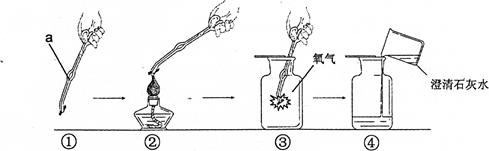
�Ը�ʵ����з������ش�
��1��ͼ��������a�������� ��
��2����ȼ�������ĽǶȷ�����ͼ�ڼ���ľ̿��Ŀ���� ��
��3��ͼ����Ϩ��ƾ��ƵIJ����� ��
��4��ͼ����ľ̿��������ȼ�ձ��ڿ�����ȼ��Ҫ���ң�˵���� ��
��5��ͼ��������ʵ������е���ͼ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������벻��ˮ��Ϊ����ʵ�����˽�ˮ��ij��ѧ�о���ѧϰС��Ը�������ˮ�ʵ�״��������صĵ����о���
��ȡˮ�������ú���ˡ�Ϊ��ʹ��������ٳ������������һ���Լ��� ��]
����Ҫ����ϻƺ�ˮ����Ӳˮ������ˮ�����õ������� ��
����Ҫ�ⶨ�ϻƺ�ˮ�����ȣ���ѡ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���ѧ�����ǵ����彡�������������������еĹ�ϵ��
��1������ʳ���и��������ʵ��� ������ţ���
A������ B������ C������
��2��ˮ���˼�һ����������������ģ����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3����ĿǰΪֹ������ʹ�õ�ȼ����Ҫ��ú�� ����Ȼ���Ȼ�ʯȼ�ϣ�������Ȼ������Ҫ�ɷ�Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʦ����ͼ��ʾװ��Ϊͬѧ������ʾʵ�顣��֪��װ�����������á�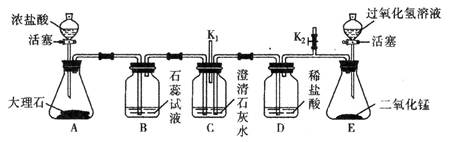
�Ŵ�A�еĻ�������������Ũ���ᣬ�ر�A�еĻ�����С����Ϊͨ��B�е�������˵��������̼��ˮ��Ӧ������̼�ᡣ����Ϊ���Ŀ��� (ѡ���������������)��˵��ԭ�� ��
��һ��ʱ����ɼ�K2��E�еĻ������������Ĺ���������Һ������ƿ�ر�E�еĻ��������ɼ�K2��C�п��ܳ��ֵ������� ��E�з�Ӧ�Ļ�ѧ����ʽΪ ��
������ʵ������У�C�в�����K����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��9�֣�������ճ�����������벻��ˮ��
��1����ѧ����ͬѧ�Ƿ����о�ˮ�ľ�������ɣ�װ����ͼ��������һ��ͬѧ�����Ƽ���ˮ����ͼ����ʾ����������ˮ�����л���̿�������� ��������ij���ˮ���Ȫˮ������ˮ������ˮ����ˮ�����ڴ��������_________��
��2��ʵ�����ͨ��һ��ʱ����Թ�b�в����������� (д��ѧʽ)����ʵ��˵��ˮ���� ��ɵģ��÷�Ӧ�ķ���ʽ�� ��
��3������ˮ��Ӳˮ������ˮ�����õ������� ��������ʹӲˮ������һ�ֳ��÷����� ��
��4�����������е����������ڽ�Լ��ˮ���� ����ѡ����
| A��ϴ�˵�ˮ�������� | B��δ����Ŀ�Ȫˮ���ֵ��� |
| C���ò���ϵ���ˮ��ϴ��� | D��ϴ��ʹ��ϴ��Һʱ��ʱ�ر�ˮ��ͷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣�ˮ���˼�һ����������������ģ�Ϊ����������
���õĿɳ�����չ������Ӧ���˽��й�ˮ��һЩ֪ʶ������ش�
��1����ͼ�ǵ��ˮʵ��װ�á���ʵ������У��Թ�1������������
____________��д��ˮ��ͨ�������·�Ӧ�Ļ�ѧ����ʽ___________________��
��Ȼˮ�к����������ʣ����������������������˺�����ȷ���������
���о����̶���ߵķ�����___________________��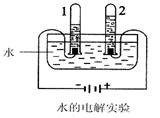
��3��Ӳˮ����������������ܶ��鷳�������п���_________________������Ӳˮ����ˮ��
��4�����������ʷֱ��������ˮ�У��ò��������Ͻ��裬���γ���ɫ��Һ����_______________������ţ���
| A���۱ʻ� | B������ͭ | C������ | D��ʳ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����д����л�ѧ��ͨ��ѧϰ�Ѿ�֪����
�ٿ������������Ϊ78%������Ļ�ѧʽ�� ��
�ڻ���̿����ȥ����������ζ�������û���̿�� �ԡ�
���ڳ���ʱ������ʳ�λ�ʳ��ˮ����ú���Ļ����ϣ�����ͳ� ɫ��
��ũҵ��������һ�ֳ��õĵ��ʣ���ѧʽ��CO��NH2��2������ ��Ԫ����ɣ����ص�Ħ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������壺�����������������۶�������һ����̼���ݶ�����̼������
��ѡ����գ�
��1���γ��������Ҫ�ɷ��ǣ�����ţ���ͬ�� ��
��2������������ˮ���� ��
��3������Ϊһ����Ҫ������Ⱦ����ȼ�ϵ��� ��
��4����ұ��ҵ�����仹ԭ�������������� ��
��5����������ѪҺ��Ѫ�쵰��ϵ��ж������� ��
��6�����ȼ��ʱ�����������������_________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com