

| 12.5g |
| 250g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?���ݣ��ꡢѩ���γɺͽ�����������ա��ܽ��˿�����SO����������������ʣ��γ���pHС��5.6�Ľ�ˮ��Ϊ���꣬��ش������й���������⣺
��2012?���ݣ��ꡢѩ���γɺͽ�����������ա��ܽ��˿�����SO����������������ʣ��γ���pHС��5.6�Ľ�ˮ��Ϊ���꣬��ش������й���������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ꡢѩ���γɺͽ�����������ա��ܽ��˿�����SO2��������������ʣ��γ���pHС��![]() 5.6�Ľ�ˮ��Ϊ���꣬��ش������й���������⣺
5.6�Ľ�ˮ��Ϊ���꣬��ش������й���������⣺
��l��Ҫ�ⶨij�زɼ�������ˮ�Ƿ�Ϊ���꣬�����ṩ���Լ�����ֽ������ȡ��
A����ɫʯ����ֽ B����ɫʯ����Һ C����ɫ��̪�Լ� D��pH![]() ��ֽ
��ֽ
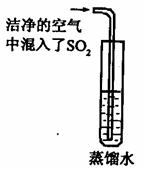
��2����֪ CO2�ı�����Һ������pH��С�� 5.6��ͨ��������CO2�ĺ������� SO2�ĺ����������Ƿ����������е� SO2�϶�Ϊ�γ��������Ҫԭ��֮һ����ͬѧ��ͨ������ͼ��ʵ��Ѱ��֤�ݣ�����Ϊ��һ����ʵ�鲽���Dzⶨ ��ֻҪ���� ��ʵ�������Ϳ��϶�SO2����ˮ���γ�����Ŀ��ܡ�
��3����֪SO2�� CO2�����ѧ���������Ƶģ�������ƣ�CaSO3���ǰ�ɫ������ˮ����������Ĺ��塣ijͬѧ��һ������Ʒ�еμ� CaCl2,��Һ���۲쵽��![]() ��������������NaOH��Һ���а�ɫ����������������ʵ������У����в����ܷ����Ļ�ѧ��Ӧ��
��������������NaOH��Һ���а�ɫ����������������ʵ������У����в����ܷ����Ļ�ѧ��Ӧ��
A SO2��CaCl2��H2O== CaSO3��ʮ2HCI
B��SO2��2NaOH==Na2SO3��H2O
C��![]() Na2SO3��CaCl2= CaSO3����2NaCI
Na2SO3��CaCl2= CaSO3����2NaCI
D��H2SO3 ��2NaOH= Na2SO3��2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012����б�ҵ��ѧ���ԣ��㽭���ݾ�����ѧ���������� ���ͣ������
��8�֣��ꡢѩ���γɺͽ�����������ա��ܽ��˿�����SO2��������������ʣ��γ���pHС��5.6�Ľ�ˮ��Ϊ���꣬��ش������й���������⣺
��l��Ҫ�ⶨij�زɼ�������ˮ�Ƿ�Ϊ���꣬�����ṩ���Լ�����ֽ������ȡ��
A����ɫʯ����ֽ B����ɫʯ����Һ C����ɫ��̪�Լ� D��pH��ֽ
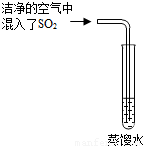
��2����֪ CO2�ı�����Һ������pH��С�� 5.6��ͨ��������CO2�ĺ������� SO2�ĺ����������Ƿ����������е� SO2�϶�Ϊ�γ��������Ҫԭ��֮һ����ͬѧ��ͨ����ͼ��ʵ��Ѱ��֤�ݣ�����Ϊ��һ����ʵ�鲽���Dzⶨ ��ֻҪ���� ��ʵ�������Ϳ��϶�SO2����ˮ���γ�����Ŀ��ܡ�
��3����֪SO2�� CO2�����ѧ���������Ƶģ�������ƣ�CaSO3���ǰ�ɫ������ˮ����������Ĺ��塣ijͬѧ��һ������Ʒ�еμ� CaCl2,��Һ���۲쵽�л�������������NaOH��Һ���а�ɫ����������������ʵ������У����в����ܷ����Ļ�ѧ��Ӧ��
A��SO2��CaCl2��H2O="=" CaSO3��ʮ2HCI
B��SO2��2NaOH==Na2SO3��H2O
C��Na2SO3��CaCl2= CaSO3����2NaCI
D��H2SO3��2NaOH= Na2SO3��2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ�п����� ���ͣ������
�ꡢѩ���γɺͽ�����������ա��ܽ��˿�����SO2��������������ʣ��γ���pH С�� 5.6�Ľ�ˮ��Ϊ���꣬��ش������й���������⣺
5.6�Ľ�ˮ��Ϊ���꣬��ش������й���������⣺
��1��Ҫ�ⶨij�زɼ�������ˮ�Ƿ�Ϊ���꣬�����ṩ���Լ�����ֽ������ȡ��
A����ɫʯ����ֽ
B����ɫʯ����Һ
C����ɫ��̪�Լ�
D��pH ��ֽ
��ֽ
��2����֪ CO2�ı�����Һ������pH ��С�� 5.6��ͨ��������CO2�ĺ�������SO2�ĺ����������Ƿ����������е� SO2�϶�Ϊ�γ��������Ҫԭ��֮һ����ͬѧ��ͨ����ͼ��ʵ��Ѱ��֤�ݣ�����Ϊ��һ����ʵ�鲽���Dzⶨ ��ֻҪ���� ��ʵ�������Ϳ��϶�SO2����ˮ���γ�����Ŀ��ܡ�
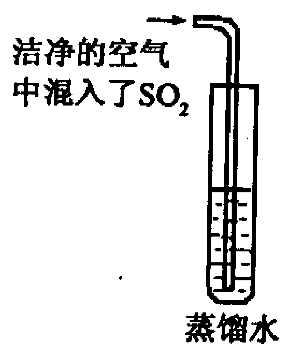
 ��������������NaOH��Һ���а�ɫ����������������ʵ������У����в����ܷ����Ļ�ѧ��Ӧ��
��������������NaOH��Һ���а�ɫ����������������ʵ������У����в����ܷ����Ļ�ѧ��Ӧ�� �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com