
 4Al+3O2”ü£®ĘäÖŠ£¬±ł¾§ŹÆ×÷ČŪ¼Į£¬æÉŹ¹Al2O3øüŅץė½ā³É×ŌÓÉĄė×Ó£®±ł¾§ŹÆµÄ»ÆѧŹ½ĪŖNa3AlF6£¬ŌņĘäÖŠ·śŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ______£®
4Al+3O2”ü£®ĘäÖŠ£¬±ł¾§ŹÆ×÷ČŪ¼Į£¬æÉŹ¹Al2O3øüŅץė½ā³É×ŌÓÉĄė×Ó£®±ł¾§ŹÆµÄ»ÆѧŹ½ĪŖNa3AlF6£¬ŌņĘäÖŠ·śŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ______£®
 3CO2+4H2O£»
3CO2+4H2O£» 3CO2+4H2O£»
3CO2+4H2O£» £¬
£¬


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2008?Ģ©ÖŻ£©ČēĶ¼ŹĒA”¢BĮ½ÖÖĪļÖŹµÄČܽā¶ČŃĪĻߣ®øł¾ŻČēĶ¼ĖłŹ¾»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø2008?Ģ©ÖŻ£©ČēĶ¼ŹĒA”¢BĮ½ÖÖĪļÖŹµÄČܽā¶ČŃĪĻߣ®øł¾ŻČēĶ¼ĖłŹ¾»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2009ğȫ¹śÖŠæ¼»ÆѧŹŌĢā·ÖĄą»ć±ąæ¼µć2 »ÆѧŹµŃ黳±¾²Ł×÷ ĢāŠĶ£ŗĢīæÕĢā
(2008”¤Ģ©ÖŻ)ĻĀĶ¼ŹĒŠ”Ć·ÅäÖĘ100gČÜÖŹÖŹĮæ·ÖŹżĪŖ12£„µÄNaClČÜŅŗµÄŹµŃé²Ł×÷Ź¾ŅāĶ¼ČēĶ¼ĖłŹ¾£ŗ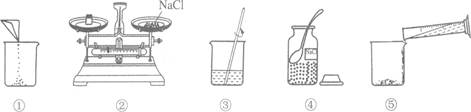
(1)ÉĻĶ¼ÖŠµÄ²£Į§ŅĒĘ÷·Ö±šŹĒ¹ćæŚĘ攢ĮæĶ²”¢ÉÕ±ŗĶ_______________”£
(2)Öø³öĶ¼ÖŠµÄŅ»“¦“ķĪó²Ł×÷___________________________________”£
(3)ÅäÖĘŹ±Ó¦Ń”Ōń_______mL(10mL»ņ50mL»ņ100mL)µÄĮæĶ²ĮæČ”ĖłŠčŅŖµÄĖ®”£
(4)ÓĆÉĻŹöĶ¼Ź¾µÄŠņŗűķŹ¾ÅäÖĘČÜŅŗµÄ²Ł×÷Ė³Šņ___________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com