
| ���� | ���� | |||||||||||||||||
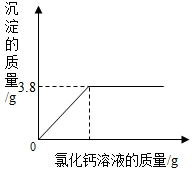
| ʵ����� | I��ȡ������100g�������ձ�����ʢ20g����õ�̼������Һ�� II���������ձ��зֱ������������������ͬ������Һ������ͬ��������Һ�� III����ַ�Ӧ���ٷֱ�����ձ���ʣ����Һ�� | I��ȡ������100g��һ���ձ���ʢ20g����õ�̼������Һ�� II���������ձ��е���������������Ϊ20%���Ȼ�����Һ��ֱ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ����ɡ����أ� III�����Ƽ����Ȼ�����Һ�����������������ϵ����ͼ�� | ||||||||||||||||
| ���õ����ݻ��ϵͼ |
|  ��֪������Ӧ�Ļ�ѧ����ʽΪ�� Na2CO3+CaCl2�TCaCO3��+2NaCl |
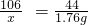
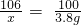



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | ���� | |||||||||||||||||
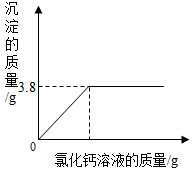
| ʵ����� | I��ȡ������100g�������ձ�����ʢ20g����õ�̼������Һ�� II���������ձ��зֱ������������������ͬ������Һ������ͬ��������Һ�� III����ַ�Ӧ���ٷֱ�����ձ���ʣ����Һ�� |
I��ȡ������100g��һ���ձ���ʢ20g����õ�̼������Һ�� II���������ձ��е���������������Ϊ20%���Ȼ�����Һ��ֱ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ����ɡ����أ� III�����Ƽ����Ȼ�����Һ�����������������ϵ����ͼ�� | ||||||||||||||||
| ���õ����ݻ��ϵͼ |
|
 ��֪������Ӧ�Ļ�ѧ����ʽΪ�� Na2CO3+CaCl2�TCaCO3��+2NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�걱���з�̨���п���ѧ��ģ�Ծ��������棩 ���ͣ������
| ���� | ���� | |||||||||||||||||
| ʵ����� | I��ȡ������100g�������ձ�����ʢ20g����õ�̼������Һ�� II���������ձ��зֱ������������������ͬ������Һ������ͬ��������Һ�� III����ַ�Ӧ���ٷֱ�����ձ���ʣ����Һ�� | I��ȡ������100g��һ���ձ���ʢ20g����õ�̼������Һ�� II���������ձ��е���������������Ϊ20%���Ȼ�����Һ��ֱ�����ٲ�������Ϊֹ�����ˡ�ϴ�ӡ����ɡ����أ� III�����Ƽ����Ȼ�����Һ�����������������ϵ����ͼ�� | ||||||||||||||||
| ���õ����ݻ��ϵͼ |
| 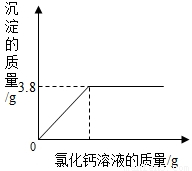 ��֪������Ӧ�Ļ�ѧ����ʽΪ�� Na2CO3+CaCl2�TCaCO3��+2NaCl |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com