
КөСйКТјИҝЙУГKMnO4ЈЁ»тKClO3әНMnO2өД»мәПОпЈ©ФЪјУИИМхјюПВК№Жд·ЦҪвЦЖИЎСхЖшЈ¬ТІҝЙУГПВНјЧ°ЦГЈ¬НЁ№э·ЦҪв№эСх»ҜЗвЈЁH2O2Ј©АҙЦЖИЎСхЖшЎЈЗлДгёщҫЭТСС§»ҜС§ЦӘК¶әНҫӯСй»ШҙрПВБРОКМвЈә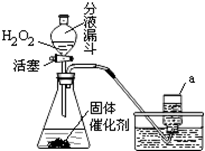
ЈЁ1Ј©Рҙіц№эСх»ҜЗв·ЦҪвөД»ҜС§·ҪіМКҪЈә
ЈЁ2Ј©РҙіцЙПНјЧ°ЦГЦРТЗЖчaөДГыіЖЈә
ЈЁ3Ј©УлёЯГМЛбјШЈЁ»тВИЛбјШәН¶юСх»ҜГМөД»мәПОпЈ©ЦЖИЎСхЖшПаұИЈ¬УГ№эСх»ҜЗвЦЖИЎСхЖшөДУЕөгКЗЈә
ЈЁ4Ј©КХјҜСхЖшөД·Ҫ·Ё»№ҝЙУГ ·ЁЈ¬ДгСЎФсҙЛ·Ҫ·ЁөДАнУЙКЗЈә
ЈЁ5Ј©ЙПНј·ўЙъЧ°ЦГ»№ҝЙТФУГУЪЦЖИЎөДЖшМеУР ЎЈ
ІўРҙіцЦЖИЎёГЖшМеөД»ҜС§·ҪіМКҪ
ЈЁ1Ј© 2H2O2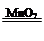 2H2O + O2Ўь
2H2O + O2Ўь
ЈЁ2Ј© јҜЖшЖҝ
ЈЁ3Ј© І»РијУИИ
ЈЁ4Ј©ПтЙПЕЕҝХЖш·Ё Ј¬ O2өДГЬ¶ИұИҝХЖшҙу
ЈЁ5Ј©H2»тCO2ЎЈ Zn+H2SO4=ZnSO4+H2Ўь CaCO3+2HCl=CaCl2+H2O+ CO2Ўь
ҪвОцКФМв·ЦОцЈәЈЁ1Ј©№эСх»ҜЗвФЪ¶юСх»ҜГМөДҙЯ»ҜЧчУГПВҝЙЙъіЙЛ®әНСхЖшЈ¬Жд»ҜС§·ҪіМКҪОӘ 2H2O2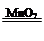 2H2O + O2ЎьЈ»
2H2O + O2ЎьЈ»
ЈЁ2Ј©јҜЖшЖҝКЗіхЦР»ҜС§іЈјыТЗЖчЈ¬УГУЪКХјҜЎўЦьҙжЖшМеЈ¬ТЗЖчaОӘјҜЖшЖҝЎЈ
ЈЁ3Ј©ёЯГМЛбјШЈЁ»тВИЛбјШәН¶юСх»ҜГМөД»мәПОпЈ©ЦЖИЎСхЖшРиТӘјУИИЈ¬¶ш№эСх»ҜЗвФЪ¶юСх»ҜГМөДҙЯ»ҜЧчУГПВҝЙіЈОВПВҫНҝЙТФҪшРРЈ¬№К№эСх»ҜЗвөДУЕөгОӘІ»РијУИИЈ¬ҪЪФјДЬФҙЎЈ
ЈЁ4Ј©ёГНјІЙУГЕЕЛ®·ЁКХјҜСхЖшЈ¬СхЖш»№ҝЙТФІЙУГПтЙПЕЕҝХЖш·ЁКХјҜЈ¬ФӯТтКЗ O2өДГЬ¶ИВФҙуУЪҝХЖшөДГЬ¶ИЎЈ
ЈЁ5Ј©ёГЧ°ЦГҝЙУГУЪ№ММеУлТәМеіЈОВПВ·ҙУҰЦЖИЎЖшМеЈ¬ЗвЖшәН¶юСх»ҜМј¶јҝЙТФУГёГЧ°ЦГЦЖИЎЈ¬·ҙУҰөД»ҜС§·ҪіМКҪОӘZn+H2SO4=ZnSO4+H2ЎьЎўCaCO3+2HCl=CaCl2+H2O+ CO2ЎьЎЈ
ҝјөгЈәҝјІйіЈјыЖшМеөДЦЖИЎТФј°Ч°ЦГөДСЎФсЎЈ
өгЖАЈә·ҙУҰЧ°ЦГөДСЎФс·ЦОӘЈә№М№МјУИИРНЎў№МТәІ»јУИИРНЈ¬КХјҜ·Ҫ·ЁТӘёщҫЭЖшМеөДИЬҪвРФәНГЬ¶ИҝјВЗЈ¬Рҙ·ҪіМКҪТӘЧўТвТ»Рҙ¶юЕдИэЧўГчЛДөИәЕЎЈ


 ҝОМГИ«ҪвЧЦҙКҫд¶ОЖӘХВПөБРҙр°ё
ҝОМГИ«ҪвЧЦҙКҫд¶ОЖӘХВПөБРҙр°ё ІҪІҪёЯҝЪЛгМвҝЁПөБРҙр°ё
ІҪІҪёЯҝЪЛгМвҝЁПөБРҙр°ё өгҫҰРВҪМІДИ«ДЬҪв¶БПөБРҙр°ё
өгҫҰРВҪМІДИ«ДЬҪв¶БПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә2012-2013С§ДкәюДПКЎҫЕДкј¶12ФВФВҝј»ҜС§КФҫнЈЁҪвОц°жЈ© МвРНЈәМоҝХМв
КөСйКТјИҝЙУГKMnO4ЈЁ»тKClO3әНMnO2өД»мәПОпЈ©ФЪјУИИМхјюПВК№Жд·ЦҪвЦЖИЎСхЖшЈ¬ТІҝЙУГПВНјЧ°ЦГЈ¬НЁ№э·ЦҪв№эСх»ҜЗвЈЁH2O2Ј©АҙЦЖИЎСхЖшЎЈЗлДгёщҫЭТСС§»ҜС§ЦӘК¶әНҫӯСй»ШҙрПВБРОКМвЈә
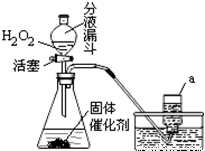
ЈЁ1Ј©Рҙіц№эСх»ҜЗв·ЦҪвөД»ҜС§·ҪіМКҪЈә
ЈЁ2Ј©РҙіцЙПНјЧ°ЦГЦРТЗЖчaөДГыіЖЈә
ЈЁ3Ј©УлёЯГМЛбјШЈЁ»тВИЛбјШәН¶юСх»ҜГМөД»мәПОпЈ©ЦЖИЎСхЖшПаұИЈ¬УГ№эСх»ҜЗвЦЖИЎСхЖшөДУЕөгКЗЈә
ЈЁ4Ј©КХјҜСхЖшөД·Ҫ·Ё»№ҝЙУГ ·ЁЈ¬ДгСЎФсҙЛ·Ҫ·ЁөДАнУЙКЗЈә
ЈЁ5Ј©ЙПНј·ўЙъЧ°ЦГ»№ҝЙТФУГУЪЦЖИЎөДЖшМеУР ЎЈ
ІўРҙіцЦЖИЎёГЖшМеөД»ҜС§·ҪіМКҪ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈәОдәә МвРНЈәөҘСЎМв
| AЈ®ўЪўЬўЭ | BЈ®ўЩўЪўЬўЭ | CЈ®ўЪўЫўЬўЭ | DЈ®ўЪўЬ |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәіхЦР»ҜС§ АҙФҙЈә МвРНЈә
КөСйКТјИҝЙУГKMnO4ЈЁ»тKClO3әНMnO2өД»мәПОпЈ©ФЪјУИИМхјюПВК№Жд·ЦҪвЦЖИЎСхЖшЈ¬ТІҝЙУГПВНјЧ°ЦГЈ¬НЁ№э·ЦҪв№эСх»ҜЗвЈЁH2O2Ј©АҙЦЖИЎСхЖшЎЈЗлДгёщҫЭТСС§»ҜС§ЦӘК¶әНҫӯСй»ШҙрПВБРОКМвЈә
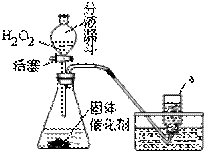
ЈЁ1Ј© Рҙіц№эСх»ҜЗв·ЦҪвөД»ҜС§·ҪіМКҪЈә
ЈЁ2Ј© РҙіцЙПНјЧ°ЦГЦРТЗЖчaөДГыіЖЈә
ЈЁ3Ј© УлёЯГМЛбјШЈЁ»тВИЛбјШәН¶юСх»ҜГМөД»мәПОпЈ©ЦЖИЎСхЖшПаұИЈ¬УГ№эСх»ҜЗвЦЖИЎСхЖшөДУЕөгКЗЈә
ЈЁ4Ј© КХјҜСхЖшөД·Ҫ·Ё»№ҝЙУГ ·ЁЈ¬ДгСЎФсҙЛ·Ҫ·ЁөДАнУЙКЗЈә
ЈЁ5Ј©ЙПНј·ўЙъЧ°ЦГ»№ҝЙТФУГУЪЦЖИЎөДЖш
МеУР ЎЈ
ІўРҙіцЦЖИЎёГЖшМеөД»ҜС§·ҪіМКҪ
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com