
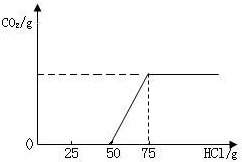
 ��2005?���ݣ��о���ѧϰ���⣺ʵ�����о��õ�NaOH�ı��ʳ̶�
��2005?���ݣ��о���ѧϰ���⣺ʵ�����о��õ�NaOH�ı��ʳ̶�| Na2CO3������/g | 5.3g 5.3g |
| ����NaOH������/g | 4.0g 4.0g |
| NaOH�ı��ʳ̶ȣ�������������ʾ�� | 66.7% 66.7% |
| 106 |
| x |
| 36.5 |
| (75g-50g)��7.3% |
| 80 |
| y |
| 106 |
| 5.3g |
| 4g |
| 7.3g-5.3g+4g |
| Na2CO3������/g | 5.3g |
| ����NaOH������/g | 4.0 g |
| NaOH�ı��ʳ̶ȣ�������������ʾ�� | 66.7% |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 12 |
| 222 |
| 12 |
| 222 |
| �� �� | �� �� | ���ۣ���ѧ����ʽ�� | ||||||||
ȡ��ɫ��ĩ�����Թ��м�ǿ�ȣ�������������ͨ�����ʯ��ˮ�� ȡ��ɫ��ĩ�����Թ��м�ǿ�ȣ�������������ͨ�����ʯ��ˮ�� |
��ĩ�ɺ�ɫ��ɺ�ɫ��ʯ��ˮ����� ��ĩ�ɺ�ɫ��ɺ�ɫ��ʯ��ˮ����� |
2CuO+C
2CuO+C
|
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com