��2013?��������ģ������A��G�����������⡢̼�������ơ����е����ֻ�����Ԫ����ɣ�
��1��A��B��C���������A��B��Ӧ�IJ������ʹ��ɫʯ����Һ��ɺ�ɫ��B��C���Ϻ�IJ���D����ʹ��ɫ��̪��Һ��죬��B�Ļ�ѧʽ��
CO2
CO2
��
��2��E��һ�ֳ�����Һ��ȼ�ϣ��������м���������E������Ϊ����ȼ�ϣ�E��������ȼ�տ�����A��B����Eȼ�յĻ�ѧ����ʽΪ
��
��3��F�ڹ�ҵ�Ϲ㷺���ڲ�������ֽ����֯��ϴ�Ӽ���������F��G��Ӧ������������A����F���׳���
����
����
��F��G��Ӧ�Ļ�ѧ����ʽ��
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
��
��4��D���к�ijЩ������ˮ�к��е�G��D��G��Ӧ�Ļ�ѧ����ʽΪ
Ca��OH��2+H2SO4=CaSO4+2H2O
Ca��OH��2+H2SO4=CaSO4+2H2O
��
��5��D��F������������ˮ������ѧ��Ӧ��д��������Һ�����ʵ����п�����ɣ�
NaOH��NaOH��Ca��OH��2��NaOH��Na2CO3
NaOH��NaOH��Ca��OH��2��NaOH��Na2CO3
���ѧʽ����




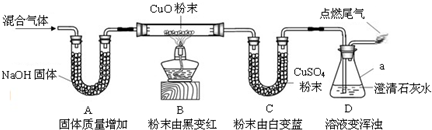
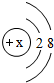

 ����ȸʯ������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]������һ����Ҫ��ͭ��ʯԭ�ϣ����ȿɷֽ�Ϊ���ֳ����Ļ��������ת����ϵ��ͼ������A�������Һ̬���ʣ�B�Ǻ�ɫ���壬F��GΪ�������ʣ������ƶϻش��������⣺
����ȸʯ������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]������һ����Ҫ��ͭ��ʯԭ�ϣ����ȿɷֽ�Ϊ���ֳ����Ļ��������ת����ϵ��ͼ������A�������Һ̬���ʣ�B�Ǻ�ɫ���壬F��GΪ�������ʣ������ƶϻش��������⣺