

���� ��1�������ܳ�ȥ�����Թ������ʣ�����̿���������ԣ�����ͭ�����ؽ����Σ��������ж�������ˮ�Dz��������ʵ�ˮ���ݴ˽��
��2�����ݵ��ˮʵ�������ͽ��۷����ش�
��3������������������ԭ���������㣻
��4�����ݷ���ˮ����ȴ��Ӳˮ����ˮ���
��� �⣺
��1��A����ˮ�м�������������������Ŀ�����������ȷ��
B�������ܳ�ȥ�����Թ������ʣ��ʲ���٢ڿɳ�ȥ���������ʣ���ȷ��
C������̿���������ԣ��ܳ�ȥˮ�е�ɫ�غ���ζ����ȷ��
D������ܿ���������ɱ�������������Ǹ�����أ�����
��2���ɵ��ˮ��װ�ÿ�֪�����Դ������B�Թ��ռ�������϶࣬����������B����Դ�������������������Թ��ڲ�������������
��3�������ռ����Ŀ�����ˮΪ������Ϊx
2000g��3%=��2000g-x����4%
��ã�x=500g
�ڴ˵�����ˮ�Ĺ��̲��õķ�����������
��4���÷���ˮ����ȴ��Ӳˮ����ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ��
�𰸣�
��1��D��
��2������
��3����500����A��
��4����ˮ��
���� ������Ҫ������ˮ�ľ�������⡢Ӳˮ����ˮ�������֪ʶ���ѶȲ��������й�ˮ�Ļ���֪ʶ����ǿ�й�ˮ��֪ʶ�Ĺ��ɺ��ܽᣬ�����ڽ������ϰ�⣮�����Ƿ��벻���Ե�����������Ե����ʣ�����ˮ��Ӳ�ȵķ������������У�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 С÷ͬѧ����ͼ��ʾ���ձ��м���һ�����ʣ������������Ƭ�ϵ�ʯ���ڻ���������������ǣ�������
С÷ͬѧ����ͼ��ʾ���ձ��м���һ�����ʣ������������Ƭ�ϵ�ʯ���ڻ���������������ǣ�������| A�� | ʳ�ι��� | B�� | �������ƹ��� | C�� | ������ | D�� | ����粒��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

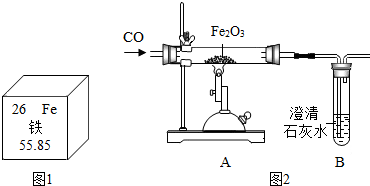
| A�� | ��Ԫ�غ���Ԫ�صĸ�����������������������ͬ | |
| B�� | ��Ԫ��λ��Ԫ�����ڱ��е������� | |
| C�� | �����Ӻ������������Ӳ㣬�����ӷ���ΪAl3+ | |
| D�� | ��ԭ�ӵ�����Ϊ55.85g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ���� | ʵ����� | ʵ������ |
| �� | ������ͨ������ʯ��ˮ�� | ����ʯ��ˮ����� |
| �� | �������ǵ�ľ������������� | �����ǵ�ľ��û�и�ȼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧ��ȤС����������ƹ��屩¶�ڿ����еı�������������о�����һС������������Ʒ��ڱ��������ʱ�䱩¶�ڿ����У���������������ʪ�����ܻ���Һ��������γɾ��壬���ձ�ɷ�ĩ����ش��������⣺
ij��ѧ��ȤС����������ƹ��屩¶�ڿ����еı�������������о�����һС������������Ʒ��ڱ��������ʱ�䱩¶�ڿ����У���������������ʪ�����ܻ���Һ��������γɾ��壬���ձ�ɷ�ĩ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com