
在一定温度下 ,将少量生石灰放入一定量的饱和石灰水中,搅拌并冷却至原来温度,下列说法正确的是( )
,将少量生石灰放入一定量的饱和石灰水中,搅拌并冷却至原来温度,下列说法正确的是( )
A.溶剂质量不变
B.溶质质量增加
C.溶液浓度不变
D.溶解度增大
 黄冈海淀全程培优测试卷系列答案
黄冈海淀全程培优测试卷系列答案科目:初中化学 来源: 题型:
某化学兴趣小组同学用Na2CO3溶液和浓HCl来研究简易灭火器的反应原理时,对废液的成分进行探究。
【推理假设】 废液的成分中一定有 ,可能有Na2CO3或盐酸.
【实验探究】 确定废液成分中是否含有Na2CO3或盐酸
请你协助兴趣小组的同学完成实验探究,并完成实验报告。
| 实验步骤 | 现象 | 结论 |
| 该废液中一定没有盐酸 | ||
| 该废液中一定含有Na2CO3 |
查看答案和解析>>
科目:初中化学 来源: 题型:
请用化学知识回答下列问题。
(1)请你用化学知识解释下列成语:
①“点石成金”:______________________________________________;
②“花香四溢”:______________________________________________;
(2)造成“煤气中毒”的气 体是_____________,为防止“煤气中毒”事件的发生,请你给居民一条建议________________________________________。
体是_____________,为防止“煤气中毒”事件的发生,请你给居民一条建议________________________________________。
查看答案和解析>>
科目:初中化学 来源: 题型:
甲、乙、丙、丁都是含有碳元素的物质,它们之间有如下转化关系:
⑴甲+丙→ 乙 ⑵乙+O2→ 丙 ⑶丁在高温下分解可得到丙
则按甲、乙、丙、丁顺序依次排列正确的一组是 ( )
A.C、CO、CO2、CaCO3 B.C、CO2、CO、Na2CO3
C.CO、C、CO2、CaCO3 D.CO2、C、CO、CaCO3
查看答案和解析>>
科目:初中化学 来源: 题型:
将黄色有毒的一氧化铅( PbO )与炭粉混合,在不同条件下能发生如下反应:
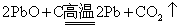

某学生设计了一个实验证明一氧化铅中含有氧元素(装置如下图所示)。试管Ⅲ中盛放澄清石灰水。
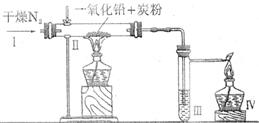 (1)通入干燥氮气的作用是将PbO和C反应生成的气体驱赶到仪器Ⅲ中。若改通空气, 则无法达到目的,原因是空气中的 等成为氧元素的来源。
(1)通入干燥氮气的作用是将PbO和C反应生成的气体驱赶到仪器Ⅲ中。若改通空气, 则无法达到目的,原因是空气中的 等成为氧元素的来源。
(2)若该学生实验时在Ⅲ处未见浑浊出现,则在Ⅱ处发生的反应是(写出化学方程式)。
(3)若要确保验证一氧化铅中一定存在氧元素的实验成功,请你对上述实验加以改进。具体方案是: 。
查看答案和解析>>
科目:初中化学 来源: 题型:
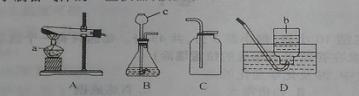 下图是实验室用语制备气体的一些仪器,按要求答题:
下图是实验室用语制备气体的一些仪器,按要求答题:
(1)写出用A制取氧气的化学方程式 ,其反应基本类
型为 ,收集装置为 。
(2)用装置B可以制备气体 (填气体化学式),发生反应的化学方程式
。
查看答案和解析>>
科目:初中化学 来源: 题型:
有t℃时浓度相同的两份KNO3溶液A和B,A为100g,B为80g,将其恒温蒸发20g水后,A刚好饱和,则关于B溶液正确的说法是( )
A.也刚好是饱和溶液
B.仍是不饱和溶液
C.是饱和溶液,并有晶体析出
D.有晶体析出剩余 不饱和溶液
不饱和溶液
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com