
���� ���ݶ�����̼�����������CH3COOH������������������Ậ�����н��
��� �⣺��CH3COOH������Ϊx��
CaCO3+2CH3COOH=��CH3COO��2 Ca+H2O+CO2��
120 44
x 1.1g
$\frac{120}{x}=\frac{44}{1.1g}$
x=3g
���Ậ��=$\frac{3g}{100g}��100%$=3%��4%-5%
���Ըó´��д����ʵ�ʺ������̱��ע�������
�𣺸ó´��д����ʵ�ʺ������̱��ע�������
���� ������Ҫ����ѧ�����û�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʱ�����������Ż��������������� | |
| B�� | ʵ��ʱ����û������İ������������� | |
| C�� | Һ������ú��й©���ʱ�����ȹر����巧�� | |
| D�� | ��¥�Ż���ʪë����ס�ڱǣ����¿������泷�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
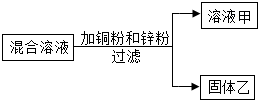 ��һ����AgNO3��Al��NO3��3�Ļ����Һ����ͭ�ۺ�п�ۣ���ַ�Ӧ����ˣ��õ���Һ�����ң���ͼ��ʾ������������˵����ȷ�ĸ���Ϊ��������
��һ����AgNO3��Al��NO3��3�Ļ����Һ����ͭ�ۺ�п�ۣ���ַ�Ӧ����ˣ��õ���Һ�����ң���ͼ��ʾ������������˵����ȷ�ĸ���Ϊ��������| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
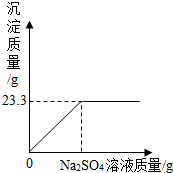 ��ȡNaCl��BaCl2�Ĺ�������32.5g������200g����ˮ����ȫ�ܽ�����
��ȡNaCl��BaCl2�Ĺ�������32.5g������200g����ˮ����ȫ�ܽ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
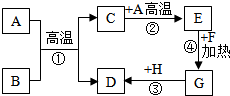 A-H�dz��л�ѧ���������ʣ���֪A�Ǻ�ɫ���嵥�ʣ�GΪ�Ϻ�ɫ���嵥�ʣ�BΪ���ɫ��ĩ��FΪ��ɫ��ĩ�����ǵ�ת����ϵ��ͼ��ʾ����ش�
A-H�dz��л�ѧ���������ʣ���֪A�Ǻ�ɫ���嵥�ʣ�GΪ�Ϻ�ɫ���嵥�ʣ�BΪ���ɫ��ĩ��FΪ��ɫ��ĩ�����ǵ�ת����ϵ��ͼ��ʾ����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢ� | C�� | �ڢ� | D�� | �٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ͼ�Ǽס��ҡ����������ʵ��ܽ�����ߣ�����˵��������ǣ�������
��ͼ�Ǽס��ҡ����������ʵ��ܽ�����ߣ�����˵��������ǣ�������| A�� | Ҫ������ͬ���������ļס����������ʵı�����Һ��Ӧ�ý��¶ȿ�����t1�� | |
| B�� | t1��ʱ�����������ʵı�����Һ������t2�棬������Һ���������������ң��ף��� | |
| C�� | Ҫ�Ӽ����ʵı�����Һ�л�þ���ף����Բ��ý��½ᾧ�ķ��� | |
| D�� | t2��ʱ��30g�����ʼ��뵽50gˮ�в��Ͻ��裬�γɵ���Һ�����ʵ���������Ϊ37.5% |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com