»ÆѧŠĖȤŠ”×éĶ¬Ń§·¢ĻÖŹµŃéĢØÉĻ°“ČēĻĀĖ³Šņ°Ś·Å×Å7Ęæ²»Ķ¬µÄĪŽÉ«ČÜŅŗ£ØČēĶ¼ĖłŹ¾£©£¬ĘäÖŠ4”¢5ŗÅŹŌ¼ĮĘæ±źĒ©ĘĘĖš£®?
[Ģį³öĪŹĢā]ÕāĮ½ĘæŹŌ¼Į·Ö±šŹĒŹ²Ć“£æ?
[²éŌÄ׏ĮĻ]
¢Ł¼īŠŌµÄŃĒĮņĖįÄĘ£ØNa
2SO
3£©ČÜŅŗ”¢Na
2CO
3ČÜŅŗ¶¼ÄÜÓėÖŠŠŌµÄCaCl
2ČÜŅŗ·¢Éśø“·Ö½ā·“Ó¦£¬²śÉś°×É«³Įµķ£®¢ŚNa
2SO
3+2HCl=2NaCl+SO
2ӟ+H
2O£®?
¢ŪCO
2ÓėSO
2¾łæÉŅŌŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē£¬SO
2ŹĒŅ»ÖÖÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壮?
[×÷³ö²ĀĻė]4”¢5ŗÅČÜŅŗæÉÄÜ·Ö±šŹĒNaOH”¢Na
2CO
3ӢNa
2SO
3»ņNaClČÜŅŗÖŠµÄŅ»ÖÖ£®
[ŹµŹ©·½°ø]ȔɣĮæ4”¢5ŗÅČÜŅŗ·Ö±šÓŚŹŌ¹ÜÖŠ£¬Č»ŗó·Ö±š½ųŠŠĻĀĮŠČż×鏵Ń飮?
ŹµŃé1£ŗŌŚĮ½Ö§ŹŌ¹ÜÖŠ·Ö±šµĪČėÉĻŹöĘßÖÖČÜŅŗÖŠµÄ
1
1
ŗÅČÜŅŗ£¬Į½ŹŌ¹ÜÖŠµÄČÜŅŗ¾ł±ä³ÉŗģÉ«£¬ĖµĆ÷4”¢5ŗÅČÜŅŗ¾ł²»æÉÄÜŹĒÉĻŹö²ĀĻėÖŠµÄ
NaCl
NaCl
ČÜŅŗ£®?ŹµŃé2£ŗŌŚĮ½Ö§ŹŌ¹ÜÖŠ·Ö±šµĪČėÉĻŹöĘßÖÖČÜŅŗÖŠµÄ3ŗÅČÜŅŗ£¬Į½ŹŌ¹ÜÖŠ¾ł²śÉś°×É«³Įµķ£¬4”¢5ŗÅČÜŅŗæÉÄÜ·Ö±šŹĒNa
2SO
3ČÜŅŗŗĶNa
2CO
3ČÜŅŗÖŠµÄŅ»ÖÖ£®Š“³öĘäÖŠŅ»øö·“Ó¦µÄ»Æѧ·½³ĢŹ½
Na2CO3+Ca£ØOH£©2ØTCaCO3”ż+2NaOH
£Ø»ņNa2SO3+Ca£ØOH£©2ØTCaSO3”ż+2NaOH£©
Na2CO3+Ca£ØOH£©2ØTCaCO3”ż+2NaOH
£Ø»ņNa2SO3+Ca£ØOH£©2ØTCaSO3”ż+2NaOH£©
£®
ŹµŃé3£ŗŌŚĮ½Ö§ŹŌ¹ÜÖŠ·Ö±šµĪČėÉĻŹöĘßÖÖČÜŅŗÖŠµÄ2ŗÅČÜŅŗ£¬ŌŚŹ¢4ŗÅČÜŅŗµÄŹŌ¹ÜÖŠÓŠ
ĪŽÉ«ĪŽĪ¶µÄĘųĢå²śÉś
ĪŽÉ«ĪŽĪ¶µÄĘųĢå²śÉś
ĻÖĻó£¬ĖµĆ÷4ŗÅŹĒNa
2CO
3ČÜŅŗ£»ŌŚŹ¢5ŗÅČÜŅŗµÄŹŌ¹ÜÖŠÓŠ
“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢå²śÉś
“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢå²śÉś
ĻÖĻó£¬ĖµĆ÷5ŗÅŹĒNa
2SO
3ČÜŅŗ£®
[ŹµŃé·“Ė¼]Ķ¬Ń§ĆĒ¾¹ż·ÖĪö£¬ČĻĪŖ4ŗÅČÜŅŗ»¹æÉÄÜŹĒ±äÖŹµÄNaOHČÜŅŗ£®ĒėÄćĄūÓĆÉĻŹöĶ¼ÖŠµÄŹŌ¼ĮÉč¼Ę¼ų¶ØŹµŃé·½°ø£¬Ķź³ÉŹµŃé±Øøę£®
| ŹµŃé²Ł×÷ |
ŹµŃéĻÖĻó |
ŹµŃé½įĀŪ |
|
|
4ŗÅČÜŅŗŹĒ²æ·Ö±äÖŹµÄNaOHČÜŅŗ£® |
25£®£Ø5·Ö£©Ä³»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§²ĪÕÕ½ĢæĘŹéÄŚČŻ£¬ŌŚŃ§Š£ŹµŃéŹŅĄļĶź³ÉĮĖŅŌĻĀĮ½øöŹµŃé£ŗ
ŹµŃéŅ»£ŗÅäÖĘČÜÖŹÖŹĮæ·ÖŹżĪŖ6%µÄNaClČÜŅŗ50g£¬°“ČēĻĀ²½Öč½ųŠŠ²Ł×÷£ŗ
ŹµŃ鶞£ŗ³ĘČ”5.0g“ÖŃĪ½ųŠŠĢį“森“ÖŃĪ³żNaClĶā£¬»¹ŗ¬ÓŠMgCl
2ӢCaCl
2ŅŌ¼°ÄąÉ³µČŌÓÖŹ£®ĪŖĮĖÓŠŠ§½«“ÖŃĪĢį“棬ŹµŃéµÄø÷²½²Ł×÷Į÷³ĢČēĶ¼2ĖłŹ¾£ŗ
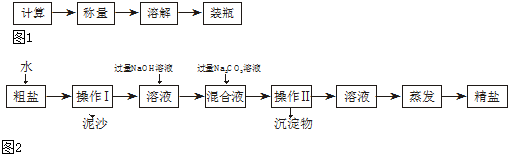
ĒėÄćøł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀŹöĪŹĢā£ŗ
£Ø1£©ÅäÖĘ50gČÜÖŹÖŹĮæ·ÖŹżĪŖ6%µÄNaClČÜŅŗ£¬ŠčNaCl
3
3
g£¬Ė®
47
47
mL£®
£Ø2£©NaClČܽā¹ż³ĢÓƵ½ĮĖ²£Į§°ō£¬ĖüµÄ×÷ÓĆŹĒ
½Į°č£¬¼ÓĖŁČܽā
½Į°č£¬¼ÓĖŁČܽā
£®
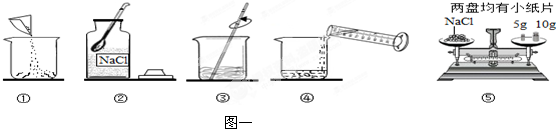
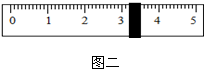
£Ø3£©“ÖŃĪĢį“æŹ±£¬²Ł×÷IµÄĆū³ĘĪŖ
¹żĀĖ
¹żĀĖ
£®
£Ø4£©Ä³Ķ¬Ń§ĖłµĆ¾«ŃĪ±ČĘäĖūĶ¬Ń§Ć÷ĻŌŅŖÉŁ£¬ŌŅņæÉÄÜŹĒ
AB
AB
£®
A£®ČܽāŹ±½«5.0g“ÖŃĪŅ»“ĪČ«²æµ¹ČėĖ®ÖŠ£¬Į¢¼“¹żĀĖ
B£®Õō·¢Ź±ÓŠŅ»Š©ŅŗĢ唢¹ĢĢ彦³ö
C£®Ģį“æŗóĖłµĆ¾«ŃĪÉŠĪ“ĶźČ«øÉŌļ£®



 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø







