ͨ����ѧѧϰ������ʶ����������ʣ���ش��������⣺
��1������Լռ��������� ������������������ ��
��2��ú��ʯ�ͺ� ����Ҫ�Ļ�ʯȼ�ϣ�����ʹ�û�ʯȼ�ϻ�ʹ������̼������ŷţ�����ȫ���ů����ѧ�Ҳ��ø��¼�������������̼��������һ����������ϣ�����һ����Ҫ�Ļ���ԭ����ϩ��C2H4����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�������ճ������е����������ϡ���̼���������� ������ĸ����ͬ����
A����Լʹ��ֽ�� B��������մ�������
C������ʹ�����ϴ� D������ʹ��һ����ֽ��
��4���Ϻ��������ڻ������ܷ�����һЩ�¼���������Դ���²��ϣ����б�������������������� ��
A��̫���ܵ����� B����Դ�ȱý��ܼ�����ʹ��
C�������ῪĻ����ʱ D�����ڽ�ͨ����ʵ�����ŷ�
E���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ磮
���𰸡�
��������1�����ݿ����ijɷּ����ɷֵ�������������жϣ�
��2�����ݻ�ʯȼ�ϵ�����������Լ����ǶԻ�����Ӱ�죻
��3�����ݡ���̼���������ǣ�
��4�����ݽ��ܻ�������������Դ���²��������ǣ�
����⣺��1�������ijɷּ����ɷֵ���������ֱ��ǣ�������78%������21%��ϡ������0.94%��������̼0.03%���������������0.03%���������������78%���ܹ���������������������
��2��ú��ʯ�ͺ���Ȼ������Ҫ�Ļ�ʯȼ�ϣ�����ʹ�û�ʯȼ�ϻ�ʹ������̼������ŷţ�����ȫ���ů����ѧ�Ҳ��ø��¼�������������̼��������һ����������ϣ�����һ����Ҫ�Ļ���ԭ����ϩ��C
2H
4����ˮ���仯ѧ����ʽΪ��
2CO
2+6H
2
C
2H
4+4H
2O��
��3������̼�����ָ���Ͷ�����̼���ŷ��������ٻ�����Ⱦ����Լʹ��ֽ�ţ�������մ�������������ʹ�����ϴ�������ʹ��һ����ֽ���������ϣ�����̼����������ѡABCD��
��4���������ܷ�����һЩ�¼���������Դ���²��ϣ�����Դ���Dz�ͬ�ڻ�ʯȼ�ϵ���Դ��̫���ܡ����ܡ����ܡ������ܡ����ܡ�ˮ�ܡ���ϫ�ܾ���������Դ��ʹ���¼���������Դ���²��ϣ��ܼ����ŷţ��Ƚ�Լ�ֻ����������ῪĻ����ʱ���������⣬��ѡC��
�ʴ�Ϊ��
��1��78%��O
2��
��2����Ȼ����2CO
2+6H
2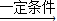
C
2H
4+4H
2O��
��3��ABCD��
��4��C��
���������⿼������ijɷּ�����ɷֵ���Ҫ��;���ر��˶�����̼��Σ�����ᳫ����̼�������������Դ���������ܻ�������������

 C2H4+4H2O��
C2H4+4H2O�� C2H4+4H2O��
C2H4+4H2O��

 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�