


���� ��1������һ����������������ʳ����Һ�IJ�������ֱ��ǣ����㡢�������ܽ⣬���ݸ���������Ҫʹ�õ��������ж����������Ƿ���Ҫ��
��2����������һ����������������ʳ����Һ�IJ���������з������
��3���������е�֪ʶ���з�������һ���¶��£���һ�������ܼ��ﲻ�����ܽ�ij�ֹ������Һ���������������������ܼ���ı�����Һ�������ܽ�Ľв�������Һ���ݴ˽��
��� �⣺��1������һ����������������ʳ����Һ�IJ�������ֱ��ǣ����㡢�������ܽ⣻������ƽ���ڳ�ȡ����ʳ�Ρ���Ͳ������ȡˮ���ձ���������ܽ�����������������ܽ�ʱ�Ľ��裬����������г�D��E�⣬������ѡ�õ�������BG��
��2������һ������������ʳ����Һ�����ȼ���������Һ����ʳ�κ�ˮ���������ٳ��������ʳ�κ���ȡˮ���������ܽ⡢װƿ����ǩ��
��3����ͼ���Կ�����B����ʣ��Ĺ��壬��Bһ���DZ�����Һ��A�м���5g������γɵ���Һ������������ػ��ܼ����ܽ⣬˵��A�Dz�������Һ��B�����¶Ⱥ�õ���C��Һ���ܱ���Ҳ���ܲ����ͣ�
x����ص��ܽ�����¶ȵ����߶�������������ش���Һ���������峣�ý��½ᾧ����ȴ�ȵı�����Һ������
�ʴ�Ϊ����1��BG����2���ܽ⣻��3����B���ڽ��½ᾧ����ȴ�ȵı�����Һ��
���� �����ѶȲ�����ȷ����һ������������������Һʵ�鲽�衢�������������ȷ�����Ĺؼ���


 ��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д�
��У��ʦ������ҵ���Ӻ����Ծ�ϵ�д� ȫ�̽��ϵ�д�
ȫ�̽��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
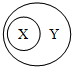 ����ѡ�����ͼʾ������ϵ���ǣ�������
����ѡ�����ͼʾ������ϵ���ǣ�������| A | B | C | D | |
| X | ���� | ����Һ | ������ | ����ʯ |
| Y | ���� | ��Һ | ������ | �Ͻ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2���壨CO������ȼ | B�� | KCl��Һ��K2CO3������ϡH2SO4 | ||
| C�� | CO2���壨HCl����ͨ��NaOH��Һ�� | D�� | MnO2���壨KCl������ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��������Zn��Fe�ֱ�������������������ϡ���ᷴӦ | |
| B�� | 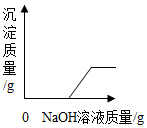 �������MgCl2�����Һ�м���������NaOH | |
| C�� | 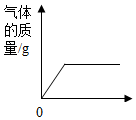 ��һƿŨ���᳨�ڷ����ڿ����� | |
| D�� |  ��һ����NaHCO3��NaCl�Ļ����Һ�еμ�ϡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ������ע����Ͳ�ڽ���ϡ��Ũ���� | |
| B�� | ��������ƽ�����������ƹ���ʱ������С�ձ��г��� | |
| C�� | CO��ԭ��������ʵ�鿪ʼʱ���ȼ��Ⱥ�ͨCO | |
| D�� | �ⶨij��Һ��pHʱ������ˮ��ʪpH��ֽ���ٽ�����Һ�ε�pH��ֽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
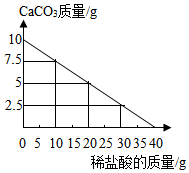 ��10gCaCO3�в��ϼ���ϡ���ᣬ�������仯��ͼ��ʾ����ش��������⣺
��10gCaCO3�в��ϼ���ϡ���ᣬ�������仯��ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com