
��6�֣�������ˮ��������̼������������Ҫ�Ļ�ѧ���ʡ�
��1��2008��9��27���ҹ�����Ա�ɹ������̫��������Ϊ�������Ա�ĺ������⣬���⺽���Ӧ���ṩ�������� ��
��2������ˮ���������У����� ������ȥˮ�в��������ʡ�
��3��ˮ��һ�ֺܺõ��ܼ������м��ּ�������������ˮ���ܼ����ƶ��ɵģ��书�ܼ���Ч�ɷ����±���ʾ��
|
�������� |
����� |
������Ư |
Ư�� |
|
���� |
��Ч����۹�������ζ |
Ưϴ���ʹɫ�ʸ����� |
����Ư���������� |
|
��Ч�ɷ� |
HCl |
H2O2 |
NaClO |
�ݱ��ش��������⣺
�� NaClO����Ԫ�صĻ��ϼ�Ϊ ��
����ʢ��������������Ư��Һ����Թ��У��������������̣��۲쵽�������� ��
�� ������顱�롰Ư�������ܻ��á�����������ײ����ж���������ͬʱ���Ȼ��ƺ�ˮ���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4������̼���������Ϊ�µ�ʱ������ȫ����̼�����ָ�����к����ٵ��������Ӷ�����̼�ر��Ƕ�����̼���ŷš��������ʽ���ϡ���̼��������е��� ����������ţ���
�ٽ����������Ķ�����̼��ʯ��ˮ����
���ٿ�˽�ҳ��������������ͨ���߳���
�۹㷺ʹ��һ���Կ��ӡ�һ�������ϴ�
���õ��ʡ�QQ�ȼ�ʱͨѶ���ߣ����ô����ӡ��
��1������ ��2������
��3����+1 �� �����ݲ���
�� 2HCl+NaClO =NaCl+ H2O+Cl2��
��4�� �ڢ�
��������
�����������1��������Ҫ��������Ϊ�������Ա�ĺ������⣬���⺽���Ӧ���ṩ��������������
��2�����˿��Գ�ȥ���������ʣ�����ˮ���������У����ù��˷�����ȥˮ�в��������ʡ�
��3�����裺NaClO����Ԫ�صĻ��ϼ�ΪX����+1��+X+��-2��=0��X=+1��
�ڡ�������Ư��Һ���к��й������⣬�����������̴���ܿ�ֽ⣬��������������ʢ��������������Ư��Һ����Թ��У��������������̣��۲쵽�������������ݲ�����
�� ������顱�롰Ư������Ϸ�Ӧ�Ļ�ѧ����ʽΪ��2HCl+NaClO =NaCl+ H2O+Cl2��
��4������̼�����ָ�����������ĵ����ʽ�����ٿ�˽�ҳ��������������ͨ���߳��С����õ��ʡ�QQ�ȼ�ʱͨѶ���ߣ����ô����ӡ�����Լ��ٶ�����̼���ŷ��������ڿ��еġ���̼�����ʽ��
���㣺��������;�����ˣ����ϼۼ�����㣻��ѧ����ʽ����̼���
�������������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ�㡣��д��ѧ����ʽҪ��ѭ����ʵ�������غ㶨������ԭ��


 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�챱���з�̨���п���ģ��ѧ�Ծ����������� ���ͣ������
������ˮ��������̼������������Ҫ�Ļ�ѧ���ʡ�
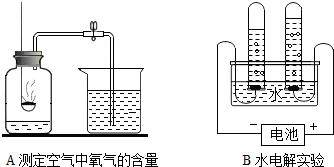
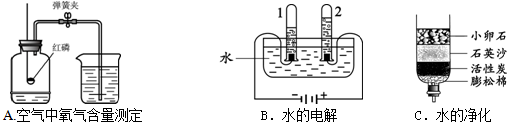
��1�����������������������ԭ���� ��
��2��ˮ��һ�ֺܺõ��ܼ������м��ּ�������������ˮ���ܼ����ƶ��ɵģ��书�ܼ���Ч�ɷ����±���ʾ��
| �������� | ����� | ������Ư | Ư�� |
| ���� | ��Ч����۹�������ζ | Ưϴ���ʹɫ�ʸ����� | ����Ư���������� |
| ��Ч�ɷ� | HCl | H2O2 | NaClO |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com