
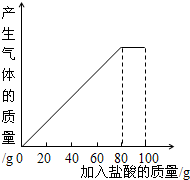
 ij����С��ͬѧ��100g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ��
ij����С��ͬѧ��100g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ��| ���� | ��һ�� | �ڶ��� | ������ |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 25 | a | 15 |
| 100 |
| 44 |
| 20g |
| x |
| 100 |
| 111 |
| 20g |
| y |
| 22.2g |
| 100g+20g-8.8g |
| 22.2g |
| 100g+20g-8.8g+k |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�������о��꼶��ѧ�ڵڶ����ʼ컯ѧ�Ծ����������� ���ͣ�������
ij����С��ͬѧ��100 g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ�� 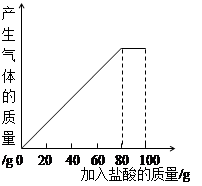
| ���� | ��һ�� | �ڶ��� | ������ |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 25 | a | 15 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�������о��꼶��ѧ�ڵڶ����ʼ컯ѧ�Ծ��������棩 ���ͣ�������
ij����С��ͬѧ��100 g�����5�μ��뵽30gʯ��ʯ��Ʒ�У� ��Ʒ�����ʼȲ�����ˮҲ�������ᷴӦ�����õ����²������ݺ�ͼ��

|
���� |
��һ�� |
�ڶ��� |
������ |
|
�������������/g |
20 |
20 |
20 |
|
ʣ����������/g |
25 |
a |
15 |
��1�����ϱ����ݷ�������2�μ��������aΪ________________g��
��2��30gʯ��ʯ��Ʒ��100gϡ���ᷴӦ�����ɶ�����̼ g��
��3�����5��ʵ�����ˣ�����CaCl2��������������Ϊ���٣�����ȷ��1%��
��4��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ��Ҫ����ˮ���ٿˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
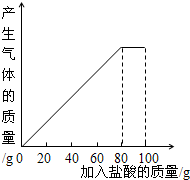
| ���� | ��һ�� | �ڶ��� | ������ |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 25 | a | 15 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�������о��꼶���£����л�ѧ�Ծ��������棩 ���ͣ������
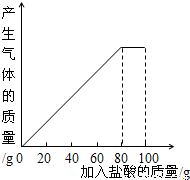
| ���� | ��һ�� | �ڶ��� | ������ |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 25 | a | 15 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com