
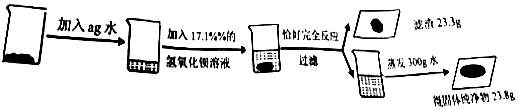
·ÖĪö £Ø1£©øł¾ŻĮņĖį¼ŲÓėĒāŃõ»Æ±µ·“Ӧɜ³ÉĮņĖį±µ³ĮµķŗĶĒāŃõ»Æ¼Ų½ųŠŠ·ÖĪö£»
£Ø2£©øł¾Ż»Æѧ·½³ĢŹ½ÖŠø÷ĪļÖŹµÄÖŹĮæ±Č·ÖĪö½ā“š£»
£Ø3£©øł¾Ż»Æѧ·½³ĢŹ½£¬½įŗĻ³ĮµķµÄÖŹĮæĄ“¼ĘĖć³öĮņĖį¼ŲµÄÖŹĮ漓æɽā“š£»
£Ø4£©øł¾ŻÖŹĮæŹŲŗć¶ØĀɼĘĖćĖłµĆČÜŅŗµÄÖŹĮ棻
£Ø6£©øł¾Ż»Æѧ·½³ĢŹ½£¬½įŗĻ³ĮµķµÄÖŹĮæĄ“¼ĘĖć³öĻūŗÄĮņĖį¼ŲѳʷµÄÖŹĮ漓æɽā“š£®
½ā“š ½ā£ŗ£Ø1£©ŅņĪŖĮņĖį¼ŲÓėĒāŃõ»Æ±µ·“Ӧɜ³ÉĮņĖį±µ³ĮµķŗĶĒāŃõ»Æ¼Ų£¬¹ŹĘä·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗK2SO4+Ba£ØOH£©2=BaSO4”ż+2KOH£»
£Ø2£©ÉčĮņĖį¼ŲµÄÖŹĮæĪŖx£®
K2SO4+Ba£ØOH£©2=BaSO4”ż+2KOH
174 233
x 23.3g
Ōņ$\frac{174}{233}=\frac{x}{23.3g}$£®½āµĆx=17.4g£®
£Ø3£©ÉčKOHµÄÖŹĮæĪŖy£®
K2SO4+Ba£ØOH£©2=BaSO4”ż+2KOH
233 56
23.3g y
Ōņ$\frac{233}{56}=\frac{23.3g}{y}$£¬y=5.6g£®
¹Ź¹¤ŅµŃłĘ·ĮņĖį¼ŲÖŠĒāŃõ»ÆÄʵÄÖŹĮæĪŖ£ŗ23.8g-5.6g=18.2g£®
¹¤ŅµŃłĘ·ÖŠĮņĖį¼ŲµÄŗ¬ĮæĪŖ$\frac{17.4g}{17.4g+18.2g}$”Į100%”Ö48.9%£®
£Ø4£©ÉčBa£ØOH£©2µÄÖŹĮæĪŖz£®
K2SO4+Ba£ØOH£©2=BaSO4”ż+2KOH
171 233
z 23.3g
Ōņ$\frac{171}{233}=\frac{z}{23.3g}$£¬½āµĆz=17.1g£®
¼ÓČėĖ®µÄÖŹĮæŹĒ£ŗ300g-£Ø$\frac{17.1g}{17.1%}$-17.1g£©=217.1g
£Ø5£©ÉčĮņĖį¼ŲµÄÖŹĮæĪŖx£®
K2SO4+Ba£ØOH£©2=BaSO4”ż+2KOH
174 112
x 119t
Ōņ$\frac{174}{112}=\frac{x}{119t}$£®½āµĆx=184.875t£®
¹Ź“ĖĮņĖį¼ŲѳʷŗĶĒāŃõ»Æ±µČÜŅŗ·“Ó¦ÖĘĒāŃõ»Æ¼Ų119t£¬ŠčøĆѳʷµÄÖŹĮæĪŖ184.875t”Ā£Ø$\frac{17.4g}{17.4g+18.2g}$”Į100%£©=378.25t£®
¹Ź“š°øĪŖ£ŗ
£Ø1£©K2SO4+Ba£ØOH£©2=BaSO4”ż+2KOH£»£Ø2£©$\frac{174}{233}=\frac{x}{23.3g}$£»£Ø3£©48.9%£»£Ø4£©217.1£»£Ø5£©378.25t£®
µćĘĄ ±¾Ģāæ¼²éĮĖĀČ»ÆÄĘÓėĢ¼ĖįÄʵĻÆѧŠŌÖŹ£¬Ķ¬Ź±æ¼²éĮĖ»Æѧ·½³ĢŹ½µÄŹéŠ“ŅŌ¼°øł¾Ż»Æѧ·½³ĢŹ½¼ĘĖćČÜŅŗµÄÖŹĮæ·ÖŹż£¬ŅŖĒóѧɜŹģĮ·ÕĘĪÕ³£¼ūĪļÖŹµÄ»ÆѧŠŌÖŹŅŌ¼°Ļą¹ŲµÄ»Æѧ·½³ĢŹ½£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢ½¾æĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÅØŃĪĖį³ØæŚ·ÅÖĆ£¬ŌŚæÕĘųÖŠŠĪ³É”°°×Īķ”± | |
| B£® | ÓĆŹ³“׳żČ„Ė®ŗųÖŠµÄĖ®¹ø | |
| C£® | ¶žŃõ»ÆĢ¼×÷ĘųĢå·ŹĮĻ | |
| D£® | øßĀÆĮ¶Ģś |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¼ĘĖćĢā
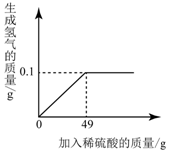 Š”ĶõĶ¬Ń§Ļė²ā¶ØijCu-ZnŗĻ½šÖŠĶµÄÖŹĮæ·ÖŹż£¬Č”Ņ»¶ØĮæŗĻ½š·ŪÄ©£¬ĻņĘäÖŠÖš½„¼ÓČėŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄĻ”ĮņĖį£¬ĖłÓĆĻ”ĮņĖįÓėÉś³ÉĒāĘųµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£®ĒėĶź³ÉĻĀĮŠ·ÖĪö¼°¼ĘĖć£ŗ
Š”ĶõĶ¬Ń§Ļė²ā¶ØijCu-ZnŗĻ½šÖŠĶµÄÖŹĮæ·ÖŹż£¬Č”Ņ»¶ØĮæŗĻ½š·ŪÄ©£¬ĻņĘäÖŠÖš½„¼ÓČėŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄĻ”ĮņĖį£¬ĖłÓĆĻ”ĮņĖįÓėÉś³ÉĒāĘųµÄÖŹĮæ¹ŲĻµČēĶ¼ĖłŹ¾£®ĒėĶź³ÉĻĀĮŠ·ÖĪö¼°¼ĘĖć£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 Š”Ć÷Ķ¬Ń§»ęÖĘĮĖČēĶ¼ĖłŹ¾A”¢BĮ½ÖÖ¹ĢĢåĪļÖŹµÄČÜŅŗ¶ČĒśĻߣŗ
Š”Ć÷Ķ¬Ń§»ęÖĘĮĖČēĶ¼ĖłŹ¾A”¢BĮ½ÖÖ¹ĢĢåĪļÖŹµÄČÜŅŗ¶ČĒśĻߣŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H+ Cl- NO3- Ba2+ | B£® | Na+ Cu2+ Cl- NO3- | ||
| C£® | Fe3+ K+ Cl- SO42- | D£® | Ba2+ NO3- Na+ Cl- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ĪĀ¶Č£Ø”ę£© | Ź¹ÓƵÄČÜÖŹ | Ź¹ÓƵÄČܼĮ | ¹Ū²ģµ½µÄŹµŃé½į¹ū |
| 20 | µā2g | ¾Ę¾«10g | Č«²æČܽā |
| 20 | µā2g | Ė®10g | ²æ·ÖČܽā |
| A£® | ĪļÖŹČܽāÄÜĮ¦µÄ“óŠ”ÓėČÜÖŹµÄŠŌÖŹÓŠ¹Ų | |
| B£® | ĪļÖŹČܽāÄÜĮ¦µÄ“óŠ”ÓėČܼĮµÄŠŌÖŹÓŠ¹Ų | |
| C£® | ĪļÖŹČܽāÄÜĮ¦µÄ“óŠ”ÓėĪĀ¶ČÓŠ¹Ų | |
| D£® | ĪļÖŹČܽāÄÜĮ¦µÄ“óŠ”ÓėČÜÖŹµÄÖŹĮæÓŠ¹Ų |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
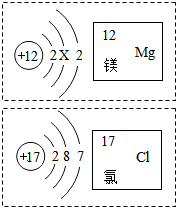 ČēĶ¼ŹĒĆ¾ŗĶĀČĮ½ÖÖŌŖĖŲµÄÓŠ¹ŲŠÅĻ¢£¬ŌņĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©
ČēĶ¼ŹĒĆ¾ŗĶĀČĮ½ÖÖŌŖĖŲµÄÓŠ¹ŲŠÅĻ¢£¬ŌņĻĀĮŠĖµ·Ø“ķĪóµÄŹĒ£Ø””””£©| A£® | Ć¾Ō×Ó½į¹¹Ķ¼ÖŠX=8 | |
| B£® | ĀČŌŖĖŲµÄŌ×ÓŠņŹżĪŖ17 | |
| C£® | Ć¾ŌŖĖŲÓėĀČŌŖĖŲ×ī±¾ÖŹµÄĒų±šŹĒ×īĶā²ćµē×ÓŹż²»Ķ¬ | |
| D£® | Ć¾ŗĶĀČ×é³É»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖMgCl2 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com