������Ի�ѧ��
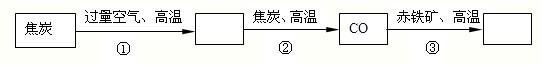
��1�������е�ȼ��
���Ż��Ƚϣ�����ú
��
��
Сľ�������������������=��������ú¯�����У������������Ŀ����
��������ֽӴ�
��������ֽӴ�
��ú����ȫȼ�ղ���һ���ж�����Ļ�ѧʽ��
CO
CO
��
����ˮ����ԭ����
ˮ�������ȣ�ʹ�¶Ƚ��͵��Ż������
ˮ�������ȣ�ʹ�¶Ƚ��͵��Ż������
������ˮ��Ĥ��ĭ���������������ʱ��������Һ�����ĭ����������չ�γ�һ��ˮĤ���������ԭ����
��������
��������
��
��2����������������;�㷺
����ͼ1����������Ʒ����Ҫ���ɽ��������Ƴɵ���
AC
AC
��

Ʒ����504˫����
�ɷ֣����ۡ�NaCl��̿��CaO�� |
ͼ3

�����������ʳ��ʱ������������м��θ��ת��Ϊ�ɱ����յ�Fe
2+����Ӧ�Ļ�ѧ����ʽΪ
Fe+2HCl�TFeCl2+H2��
Fe+2HCl�TFeCl2+H2��
����
�û�
�û�
��Ӧ���������Ӧ���ͣ���
����������װҩ���Ҫ����������
��չ
��չ
�ԣ�
��ͭ���кܺõ�
����
����
�ԣ��ʿ������ߣ�����ʪ��ұ���������ָ
Fe+CuSO4�TFeSO4+Cu
Fe+CuSO4�TFeSO4+Cu
���ѧ����ʽ����ͭ�Ϳ����е�O
2��H
2O��
CO2
CO2
������ͭ��[Cu
2��OH��
2CO
3]����Ӧ�Ļ�ѧ����ʽ��
2Cu+O2+H2O+CO2�TCu2��OH��2CO3
2Cu+O2+H2O+CO2�TCu2��OH��2CO3
��
��3�������е�ˮ����Һ
�ٴ�����Ȼˮʱ�����õĻ�������
����
����
�������ƣ�������������һ�����͵����������仯ѧʽΪ
ClO2
ClO2
��
��������500mL 0.9%��������ˮ���ܶ�Ϊ1.0g/mL������ҪNaCl������Ϊ
4.5
4.5
g�����ƹ����У���������������
���裬�ӿ��ܽ�
���裬�ӿ��ܽ�
������NaClʱ����������������̣�1g���������룩��������������ȷ����������Һ����������������
��
��
0.9%�����������������=����
��ͼ2�Ǿ�����ˮ�ļ���װ�ã�����˵����ȷ����
D
D
��
A���������ˮ�Ǵ����� B����װ���ܶ�ˮɱ������
C����װ���ܰ�Ӳˮ��Ϊ��ˮ D������̿������ˮ�е�ɫ�ؼ���ζ
��KNO
3������������������Ӫ��Һ���±��ṩ��KNO
3�ڲ�ͬ�¶�ʱ���ܽ�ȣ�
| �¶�/�� |
0 |
20 |
40 |
60 |
80 |
| �ܽ��/g |
13.3 |
31.6 |
63.9 |
110 |
169 |
a�����ϱ����ܽ��KNO
3���ܽ�����¶ȱ仯��������
���¶����߶�����
���¶����߶�����
��
b��20��ʱ����20gKNO
3����50gˮ�г���ܽ⣬������Һ������Ϊ
65.8
65.8
g��
c������60��ʱ��KNO
3������Һ210g��������20�棬������KNO
378.4
78.4
g��
��4��ʳƷ��װѧ�ʴ�
�������ز�--˻�����������հ�װ���ӳ���ʳƷ�ı����ڣ�ԭ����
�����������ž���������
�����������ž���������
��
�ڽ�N
2�����װ������������������ΪN
2�Ļ�ѧ����
�ȶ�
�ȶ�
������á����ȶ�������
��ͼ3�ǡ�504˫�������ı�ǩ�����ʴ��������⣺
a���������ٺ���
2
2
�ֵ��ʣ�
b��ȡ����˫������������ˮ�У����ã�����ϲ���Һ��pH=9������pH��ֽ�ⶨ�����������
�ò�����պȡһ���ϲ���Һ������pH��ֽ�ϣ�����ɫ���ٱ仯ʱ�ͱ���ɫ�����գ�����
�ò�����պȡһ���ϲ���Һ������pH��ֽ�ϣ�����ɫ���ٱ仯ʱ�ͱ���ɫ�����գ�����
��ͨ��CO
2���ϲ���Һ����ǣ�д�������仯�Ļ�ѧ����ʽ
H2O+CaO�TCa��OH��2
H2O+CaO�TCa��OH��2
��
Ca��OH��2+CO2�TCaCO3��+H2O
Ca��OH��2+CO2�TCaCO3��+H2O
��
c��Ϊ�ⶨ��˫������ʹ��Ч����ȡ����˫����������ͼ4��ʾ��ʵ�飬һ��ʱ������Թ���ˮ�����
��
��
21%�����������������=������������
��˫������ͬʱ���տ����е�O2��CO2��Ҳ������H2O
��˫������ͬʱ���տ����е�O2��CO2��Ҳ������H2O
��
d������ʧЧ��˫�����У��к���ɫ���壬����Ҫ��
������
������
��
e����˫�����У�NaCl�����ÿ�����
�ӿ�˫��������������O2������
�ӿ�˫��������������O2������
��




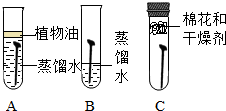 �����������ִ������ҵ������Ӧ�ü�Ϊ�ձ��һ�����ʣ�
�����������ִ������ҵ������Ӧ�ü�Ϊ�ձ��һ�����ʣ�


