
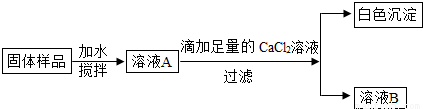
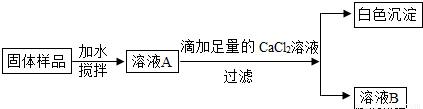
С��ͬѧ��ʵ���ҷ���һƿ���ڷ��õ�NaOH���壬������ƿNaOH����ı����������������ʵ��̽����
(1)ȡ�����������Թ��У��μ�ϡ���ᣬ�����ݲ������ɴ˿�֪��NaOH�����ѱ��ʡ�д�����������ڿ����б��ʵĻ�ѧ����ʽ ��
(2)��ͬѧΪ��һ��̽���������Ƿ���NaOH�����������ʵ�鷽����

�ٵμ�������CaCl2��ҺĿ���� ��
���ɳ����Ļ�ѧ����ʽΪ ��
��ȡ������ҺB���Թ��У��μ����� ��Һ������Ϊ ��
������ǹ�����Ʒ�� (����ڡ������ڡ�)�������ơ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������п����� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ڶ�ʮ�조��ԭ����ȫ������ѧ����ѧ���ʺ�ʵ���������������������������Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�����������п���ѧ��ģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ�����п���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com