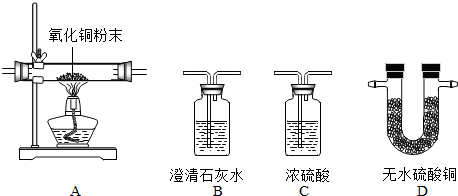
£Ø2012?ćÉŠŠĒųŅ»Ä££©£Ø1£©Ä³»ÆѧŠĖȤŠ”×éĄūÓĆĻĀĶ¼×°ÖĆĢ½¾æÖĘČ”ĘųĢåµÄŌĄķ”¢·½·Ø¼°ŠŌÖŹ£®½įŗĻ×°ÖĆĶ¼£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
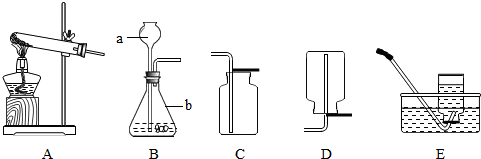
¢ŁŠ“³öĶ¼ÖŠ±źŗÅŅĒĘ÷µÄĆū³Ę£ŗa
³¤¾±Ā©¶·
³¤¾±Ā©¶·
£¬b
׶ŠĪĘæ
׶ŠĪĘæ
£®
¢ŚÓĆĖ«ŃõĖ®ŗĶ¶žŃõ»ÆĆĢÖĘČ”ŃõĘųŹ±£¬æÉŃ”ÓƵķ¢Éś×°ÖĆŹĒ
B
B
£ØĢīŠņŗÅ£©£¬æÉŃ”ÓĆC×°ÖĆŹÕ¼ÆŃõĘų£¬ĘäŌŅņŹĒ
ŃõĘųµÄĆܶȱČæÕĘų“ó
ŃõĘųµÄĆܶȱČæÕĘų“ó
£»¼ģŃéÕāĘæŃõĘųŅŃŹÕ¼ÆĀśµÄ·½·ØŹĒ
“ų»šŠĒľĢõ·ÅŌŚ¼ÆĘųĘææŚ£¬Čōø“Č¼Ö¤Ć÷Āś
“ų»šŠĒľĢõ·ÅŌŚ¼ÆĘųĘææŚ£¬Čōø“Č¼Ö¤Ć÷Āś
£®
¢ŪÓĆÕāĘæŃõĘų½ųŠŠĢśĖæŌŚŃõĘųÖŠČ¼ÉÕŹµŃé£ŗĢśĖæŌŚŃõĘųÖŠČ¼ÉÕµÄŹµŃéĻÖĻó
¾ēĮŅČ¼ÉÕ£¬»šŠĒĖÄÉä£¬Éś³ÉŗŚÉ«¹ĢĢ壬·Å³öČČĮæ
¾ēĮŅČ¼ÉÕ£¬»šŠĒĖÄÉä£¬Éś³ÉŗŚÉ«¹ĢĢ壬·Å³öČČĮæ
£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½
£®øĆŹµŃéÖŠ¼ÆĘųĘæ±»ÕØĮŃĮĖ£¬ŌŅņ
¼ÆĘųĘæ֊ƻӊ·ÅÉŁĮæĖ®»ņĘĢ»Ęɳ
¼ÆĘųĘæ֊ƻӊ·ÅÉŁĮæĖ®»ņĘĢ»Ęɳ
£®
£Ø2£©ŹµŃéŹŅĻÖÓŠ“óĄķŹÆ”¢øßĆĢĖį¼Ų”¢Ļ”ŃĪĖį”¢Ļ”ĮņĖįŗĶ×ĻÉ«ŹÆČļŹŌŅŗ¼°Ļą¹ŲµÄŅĒĘ÷ŗĶÓĆĘ·£¬Š”Ć÷Ķ¬Ń§ŅŖĶعżŹµŃéŃéÖ¤¶žŃõ»ÆĢ¼ÄÜÓėĖ®·“Ó¦µÄŠŌÖŹ£®Ēė½įŗĻÓŅĶ¼»Ų“šĻĀĮŠĪŹĢā£ŗ
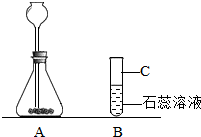
¢ŁĮ¬½ÓAŗĶB²¢½«ŹµŃé×°ÖĆĶ¼²¹³äĶźÕū£®
¢ŚAÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
CaCO3+2HClØTCaCl2 +CO2”ü+H2O
CaCO3+2HClØTCaCl2 +CO2”ü+H2O
£®
¢ŪBÖŠ¹Ū²ģµ½µÄŹµŃéĻÖĻóŹĒ
×ĻÉ«ŹÆČļŹŌŅŗ±äŗģÉ«£Ø²¢ĒŅµ¼¹ÜæŚÓŠĘųÅŻĆ°³ö£©
×ĻÉ«ŹÆČļŹŌŅŗ±äŗģÉ«£Ø²¢ĒŅµ¼¹ÜæŚÓŠĘųÅŻĆ°³ö£©
£®
¢ÜŌŚŅ»°ćĒéæöĻĀŅĒĘ÷CµÄ×÷ÓĆŹĒ
ÓĆ×÷ÉŁĮæŹŌ¼ĮµÄ·“ӦȯĘ÷£¬ŌŚ³£ĪĀ»ņ¼ÓČČŹ±Ź¹ÓĆ
ÓĆ×÷ÉŁĮæŹŌ¼ĮµÄ·“ӦȯĘ÷£¬ŌŚ³£ĪĀ»ņ¼ÓČČŹ±Ź¹ÓĆ
£®





 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø