
 ��һ�û�ѧʵ����ϣ���ʦΪÿ��ͬѧ�ֱ��ṩ��һƿ����������Һ����������1%��ϡ�������ⶨ�����ʵ����������������Ǽ���ͬѧ�����뼰������
��һ�û�ѧʵ����ϣ���ʦΪÿ��ͬѧ�ֱ��ṩ��һƿ����������Һ����������1%��ϡ�������ⶨ�����ʵ����������������Ǽ���ͬѧ�����뼰���������� ��1���ٸ���pH��ֽʹ�÷��������жϣ�
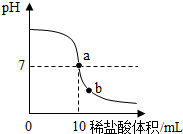
�ڸ�����ҺpH�ı仯ͼ������壬����a�ĺ��塢b����Һ�е������ӣ��������������ܶȿ���������������
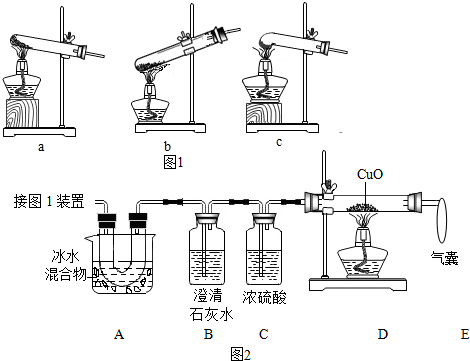
��2���������������������̼�ķ�Ӧд���������Ʊ��ʵķ���ʽ������̼���Ƶ����ʷ�����Ƴ�ȥ̼���Ƶ�ʵ�鷽����
��� �⣺��1����A����pH��ֱֽ�Ӳ������Һ�У�����Ⱦ�Լ�����������
B����pH��ֽ���ڸɾ��İ״ɰ��ϣ��ò�����պȡ����Һ����pH��ֽ�ϣ�������ȷ��
C����pH��ֽ��ʪ��ü�Һ��pHƫС����������
������ҺpH�ı仯ͼ���֪����a��ʱ����Һ��pH����7��˵�����������ƺ�����ǡ���кͣ���b��ʱ����Һ�����ԣ���������ȫ�������ᷴӦ�������Ȼ��ƣ���Һ�л���ʣ������ᣮ������Һ�е��������ǣ�Na+��H+��Ҫ������������Һ������������������֪����������������е�������֪������Ҫ��������ϡ������ܶȣ�
��2���������Ʊ��ʵ�ԭ������������������еĶ�����̼��Ӧ����Ӧ�ķ���ʽ�ǣ�CO2+2NaOH=Na2CO3+H2O������̼������������������Һ��������������Һ����Ӧ�������������ƺ�̼��ƣ���̼�ᱵ�����ȳ�ȥ������̼���ƣ���û�������µ����ʣ�����Ҫ��ȥ��Һ�б������ɵ����ʣ�ʵ�鷽���ǣ�����������ʯ��ˮ������������Һ�����ˣ�
�ʴ�Ϊ����1����B����ǡ���кͣ�Na+��H+��ϡ������ܶȣ���2��CO2+2NaOH=Na2CO3+H2O��CD��
���� �����ǿ����кͷ�Ӧ��������ҺpH�ı仯����ģ�����Ҫ֪�������мӼ������м���ʱ����ҺpH�ı仯������ȷ��ָͬʾ���ı�ɫ��Χ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����е����������� | B�� | ����������¯������pH�� | ||
| C�� | ���Ͻ��Ŵ��ȸ����Ŵ�����ʴ | D�� | ����KNO3��NH4NO3Ӫ��Ԫ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ����������� | �����Լ� |
| A | ����ˮ�����������Һ | �������� |
| B | �������ʳ�ι��� | ˮ |
| C | ϡ�������Ȼ�����Һ | ��̪��Һ |
| D | һ����̼�������̼���� | �����ʯ��ˮ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� �� | �� �� | �� �� �� �� | �� �� �� Ӧ |
| 101�桫102�� | 150�桫160�� ���� | 100.1��ֽ��ˮ��175��ֽ��CO2��CO��H2O | �� Ca��OH��2��Ӧ������ɫ������CaC2O4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ۣ�ˮ������ԭ�Ӻ���ԭ�ӹ��ɵ� | |
| B�� | ����ת���ۣ�п������п�����ת�� | |
| C�� | ���ݽṹ�ۣ��ԭ�Ӻ�������������������ͬ����ѧ������ͬ | |
| D�� | �����غ�ۣ�10mL��������40%�����ᣬ��10mLˮ������������Ϊ20% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�����顡���ڡ����� | ʵ�����顡���֡����� |
| ������ά����CƬ��������ˮ�����Һ�������еμ���ɫʯ����Һ | ��Һ���ɫ |
| ������ά����CƬ��������ˮ�����Һ���ò�����պȡ����Һ����pH��ֽ�ϣ��Ժ���� | pHС��7 |
| ֭Һ | ������������֭Һ | ����һ�ܵ���������֭Һ |
| ���� | 12 | 20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1���û�ѧ������գ�
��1���û�ѧ������գ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com