
| ŹµŃé²½Öč¼°²Ł×÷ | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ¢ŁČ”ŃłÓŚŹŌ¹ÜÖŠ£¬¼ÓČė×ćĮæÕōĮóĖ®Õńµ“£¬¾²ÖĆČ”ÉĻ²ćĒåŅŗ£¬µĪČėĪŽÉ«·ÓĢŖČÜŅŗ£» ¢Śµ¹Č„ÉĻ²ćĒåŅŗ£¬ŌŁĻņŹŌ¹Ü֊עČėĻ”ŃĪĖį | ¢ŁĪŽÉ«·ÓĢŖČÜŅŗ±äŗģ£» ¢ŚÓŠĘųÅŻ²śÉś | ²æ·Ö±äÖŹ |
| ¢ŁĪŽÉ«·ÓĢŖČÜŅŗ²»±äŗģ£» ¢ŚÓŠĘųÅŻ²śÉś | Č«²æ±äÖŹ | |
| ¢ŁĪŽÉ«·ÓĢŖŹŌŅŗ±äŗģ£» ¢ŚĆ»ÓŠĘųÅŻ²śÉś | ƻӊ±äÖŹ |
·ÖĪö £Ø2£©øł¾ŻĒāŃõ»ÆøʱäÖŹŹĒÓė¶žŃõ»ÆĢ¼·“Ó¦²śÉśĢ¼ĖįøĘŗĶĖ®Š“³öĻą¹ŲµÄ·½³ĢŹ½£»
£Ø3£©øł¾ŻĒāŃõ»ÆøʵıäÖŹ³Ģ¶Č·ÖĪöĻÖĻó£ŗ¢ŁĆ»ÓŠ±äÖŹ£¬Ö»ŗ¬ÓŠĒāŃõ»ÆøĘ£¬¼Ó·ÓĢŖŹŌŅŗ»į擵½·ÓĢŖŹŌŅŗ±ä³ÉŗģÉ«£¬¼ÓĻ”ŃĪĖįƻӊĘųÅŻ²śÉś£»
¢Ś²æ·Ö±äÖŹ£¬Ōņŗ¬ÓŠĒāŃõ»ÆøĘŗĶĢ¼ĖįøĘ£¬¼Ó·ÓĢŖŹŌŅŗ»į擵½·ÓĢŖŹŌŅŗ±ä³ÉŗģÉ«£¬¼ÓĻ”ŃĪĖįÓŠĘųÅŻ²śÉś£»
¢ŪČ«²æ±äÖŹ£¬Ö»ŗ¬ÓŠĢ¼ĖįøĘ£¬¼Ó·ÓĢŖŹŌŅŗ²»»į擵½·ÓĢŖŹŌŅŗ±ä³ÉŗģÉ«£¬¼ÓĻ”ŃĪĖįÓŠĘųÅŻ²śÉś£»
£Ø4£©¢Ł“ÓŹµŃé½įĀŪ£ŗ”°²æ·Ö±äÖŹ”±£¬æÉÖŖ¹ĢĢåÖŠŗ¬ÓŠĢ¼ĖįøĘŗĶĒāŃõ»ÆøĘČ„·ÖĪö£»
¢Ś“ÓĒāŃõ»ÆøĘĖ×³ĘŹģŹÆ»Ņ£¬Å©ŅµÉĻ³£ÓĆĄ“øÄĮ¼ĖįŠŌĶĮČĄ”¢ÅäÖĘÅ©Ņ©²Ø¶ū¶ąŅŗµČČ„·ÖĪö£»
£Ø5£©øł¾ŻĢāŅā£¬ĒāŃõ»ÆÄĘČÜŅŗÓėĮņĖį·“Ӧɜ³ÉĮņĖįÄĘŗĶĖ®£¬ÓÉĻūŗÄ5%µÄĒāŃõ»ÆÄĘČÜŅŗ80g£¬½įŗĻČÜÖŹÖŹĮæ=ČÜŅŗÖŹĮæ”ĮČÜÖŹµÄÖŹĮæ·ÖŹż”¢·“Ó¦µÄ»Æѧ·½³ĢŹ½½ųŠŠ·ÖĪö½ā“š¼“æÉ£®
½ā“š ½ā£ŗ£Ø2£©ĒāŃõ»ÆøʱäÖŹÓė¶žŃõ»ÆĢ¼·“Ó¦²śÉśĢ¼ĖįøĘŗĶĖ®£¬¹Ź·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗCa£ØOH£©2+CO2ØTCaCO3”ż+H2O£»
£Ø3£©ÓÉŹµŃé½įĀŪ£ŗ”°²æ·Ö±äÖŹ”±£¬æÉÖŖ¹ĢĢåÖŠŗ¬ÓŠĢ¼ĖįøĘŗĶĒāŃõ»ÆøĘ£»ĖłŅŌȔѳӌŹŌ¹ÜÖŠ£¬¼ÓČė×ćĮæÕōĮóĖ®Õńµ“£¬¾²ÖĆ¢ŁČ”ÉĻ²ćĒåŅŗ£¬µĪČėĪŽÉ«·ÓĢŖŹŌŅŗ£¬ĒāŃõ»ÆøĘČÜŅŗ³Ź¼īŠŌ£¬ÄÜŹ¹ĪŽÉ«·ÓĢŖŹŌŅŗ±äŗģ£»¢Śµ¹Č„ÉĻ²ćĒåŅŗ£¬ŌŁĻņŹŌ¹Ü֊עČėĻ”ŃĪĖį£¬Ļ”ŃĪĖįŗĶĢ¼ĖįøĘ£ØÓÉÓŚ¼ÓČė×ćĮæµÄÕōĮóĖ®£¬ĒāŃõ»ÆøĘČ«²æČܽāŌŚĖ®ÖŠ£©·“Ӧɜ³É¶žŃõ»ÆĢ¼ĘųĢ壻
ÓÉŹµŃé½įĀŪ£ŗ”°Č«²æ±äÖŹ”±£¬æÉÖŖ¹ĢĢåÖŠŗ¬ÓŠĢ¼ĖįøĘ£»ĖłŅŌȔѳӌŹŌ¹ÜÖŠ£¬¼ÓČė×ćĮæÕōĮóĖ®Õńµ“£¬¾²ÖĆ¢ŁČ”ÉĻ²ćĒåŅŗ£¬µĪČėĪŽÉ«·ÓĢŖŹŌŅŗ£¬ÓÉӌƻӊĒāŃõ»ÆøĘČÜŅŗ£¬ĖłŅŌĪŽÉ«·ÓĢŖŹŌŅŗ²»±äÉ«£»¢Śµ¹Č„ÉĻ²ćĒåŅŗ£¬ŌŁĻņŹŌ¹Ü֊עČėĻ”ŃĪĖį£¬Ļ”ŃĪĖįŗĶĢ¼ĖįøĘ·“Ӧɜ³É¶žŃõ»ÆĢ¼ĘųĢ壻
ÓÉŹµŃé½įĀŪ£ŗ”°Ć»ÓŠ±äÖŹ”±£¬æÉÖŖ¹ĢĢåÖŠŗ¬ÓŠÖ»ÓŠĒāŃõ»ÆøĘ£»ĖłŅŌȔѳӌŹŌ¹ÜÖŠ£¬¼ÓČė×ćĮæÕōĮóĖ®Õńµ“£¬¾²ÖĆ¢ŁČ”ÉĻ²ćĒåŅŗ£¬µĪČėĪŽÉ«·ÓĢŖŹŌŅŗ£¬ĒāŃõ»ÆøĘČÜŅŗ³Ź¼īŠŌ£¬ÄÜŹ¹ĪŽÉ«·ÓĢŖŹŌŅŗ±äŗģ£»¢Śµ¹Č„ÉĻ²ćĒåŅŗ£¬ŌŁĻņŹŌ¹Ü֊עČėĻ”ŃĪĖį£¬ÓÉӌƻӊĢ¼ĖįøĘ£¬ĖłŅŌ²»»į·“Ӧɜ³É¶žŃõ»ÆĢ¼ĘųĢ壻
¹ŹĢī£ŗÓŠĘųÅŻ²śÉś£»ÓŠĘųÅŻ²śÉś£»ĪŽÉ«·ÓĢŖŹŌŅŗ±äŗģ£»
£Ø4£©¢ŁÓÉŹµŃé½įĀŪ£ŗ”°²æ·Ö±äÖŹ”±£¬æÉÖŖ¹ĢĢåÖŠŗ¬ÓŠĢ¼ĖįøĘŗĶĒāŃõ»ÆøĘ£»ĖłŅŌȔѳӌŹŌ¹ÜÖŠ£¬¼ÓČė×ćĮæÕōĮóĖ®Õńµ“£¬¾²ÖĆ¢ŁČ”ÉĻ²ćĒåŅŗ£¬µĪČėĪŽÉ«·ÓĢŖŹŌŅŗ£¬ĒāŃõ»ÆøĘČÜŅŗ³Ź¼īŠŌ£¬ÄÜŹ¹ĪŽÉ«·ÓĢŖŹŌŅŗ±äŗģ£»¢Śµ¹Č„ÉĻ²ćĒåŅŗ£¬ŌŁĻņŹŌ¹Ü֊עČėĻ”ŃĪĖį£¬Ļ”ŃĪĖįŗĶĢ¼ĖįøĘ£ØÓÉÓŚ¼ÓČė×ćĮæµÄÕōĮóĖ®£¬ĒāŃõ»ÆøĘČ«²æČܽāŌŚĖ®ÖŠ£©·“Ӧɜ³É¶žŃõ»ÆĢ¼ĘųĢåĖ®ŗĶĀČ»ÆøĘ£»Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗCaCO3+2HClØTCaCl2+H2O+CO2”ü£»¹Ź“š°øĪŖ£ŗCaCO3+2HClØTCaCl2+H2O+CO2”ü£»
¢ŚĒāŃõ»ÆøĘĖ×³ĘŹģŹÆ»Ņ£¬Å©ŅµÉĻ³£ÓĆĄ“øÄĮ¼ĖįŠŌĶĮČĄ”¢ÅäÖĘÅ©Ņ©²Ø¶ū¶ąŅŗµČ£»¹Ź“š°øĪŖ£ŗøÄĮ¼ĖįŠŌĶĮČĄµČ£»
£Ø5£©½ā£ŗÉčŅ»¶ØĮæŹÆÓĶ²śĘ·ÖŠŗ¬H2SO4µÄÖŹĮæĪŖx£¬
2NaOH+H2SO4ØTNa2SO4+2H2O
80 98
80g”Į5% x
$\frac{80}{80g”Į5%}$=$\frac{98}{x}$ x=4.9g
“š£ŗŅ»¶ØĮæŹÆÓĶ²śĘ·ÖŠŗ¬H2SO4µÄÖŹĮæŹĒ4.9g£®
µćĘĄ ±¾Ģā×ŪŗĻŠŌ½ĻĒ棬漲éѧɜ¶ŌÓŠ¹ŲĒāŃõ»ÆøʵÄÓĆĶ¾”¢±äÖŹµÄŌŅņ¼°±äÖŹŗó²śĪļµÄĻą¹ŲŠŌÖŹµÄĄķ½āÓėÓ¦ÓĆ¼°Įé»īŌĖÓĆ½ųŠŠ·ÖĪöĪŹĢā”¢½ā¾öĪŹĢāµÄÄÜĮ¦£®


 ŠĀ»īĮ¦×ܶÆŌ±ŹīĻµĮŠ“š°ø
ŠĀ»īĮ¦×ܶÆŌ±ŹīĻµĮŠ“š°ø ĮśČĖĶ¼ŹéæģĄÖ¼ŁĘŚŹī¼Ł×÷ŅµÖ£ÖŻ“óѧ³ö°ęÉēĻµĮŠ“š°ø
ĮśČĖĶ¼ŹéæģĄÖ¼ŁĘŚŹī¼Ł×÷ŅµÖ£ÖŻ“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃéÄæµÄ | ŹµŃéÉč¼Ę |
| A | “ÓæÕĘųÖŠ»ńČ”“æ¾»µÄµŖĘų | ÓĆĮņČ¼ÉÕ³żČ„æÕĘųÖŠµÄŃõĘų |
| B | ¼ų±šÓ²Ė®ŗĶČķĖ® | ¹Ū²ģŃÕÉ«»ņµĪ¼Ó·ŹŌķĖ® |
| C | ³żČ„»ģŌŚĒāŃõ»ÆÄĘČÜŅŗÖŠµÄĢ¼ĖįÄĘČÜŅŗ | ¼Ó×ćĮæµÄĻ”ŃĪĖį |
| D | ¼ų±šĻõĖįļ§ŗĶĀČ»ÆÄĘ¹ĢĢå | ¼ÓŹŹĮæµÄĖ®Čܽā£¬²āĮæĒ°ŗóĪĀ¶Č±ä»Æ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øĘ | B£® | Ģś | C£® | µā | D£® | Šæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  Ēćµ¹ŅŗĢå | B£® | 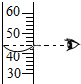 ¶ĮČ”ŹżÖµ | C£® |  ¼ÓČČŅŗĢå | D£® |  ¼ģ²éĘųĆÜŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŹµŃé²½Öč | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ȔɣĮæ·“Ó¦ŗóµÄČÜŅŗÓŚŹŌ¹ÜÖŠ£¬ĻņŹŌ¹ÜÖŠµĪ¼Ó¼øµĪĪŽÉ«·ÓĢŖČÜŅŗ£¬Õńµ“ | ČÜŅŗ²»±äÉ« | ²ĀĻė¢Ś²»ÕżČ· |
| ²ĀĻė¢ŪÕżČ· |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼īŠŌČÜŅŗÄÜŹ¹·ÓĢŖŹŌŅŗ±äŗģ£¬ĖłŅŌÄÜŹ¹·ÓĢŖŹŌŅŗ±äŗģµÄČÜŅŗŅ»¶Ø³Ź¼īŠŌ | |
| B£® | Ąė×ÓŹĒ“ųµēŗɵÄĮ£×Ó£¬ĖłŅŌ“ųµēŗɵÄĮ£×ÓŅ»¶ØŹĒĄė×Ó | |
| C£® | ÓŠ»śĪļ¶¼ŗ¬ÓŠĢ¼ŌŖĖŲ£¬ĖłŅŌŗ¬Ģ¼ŌŖĖŲµÄ»ÆŗĻĪļŅ»¶ØŹĒÓŠ»śĪļ | |
| D£® | µ„ÖŹÓÉĶ¬ÖÖŌŖĖŲ×é³É£¬ĖłŅŌÓÉĶ¬ÖÖŌŖĖŲ×é³ÉµÄĪļÖŹŅ»¶ØŹĒµ„ÖŹ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com