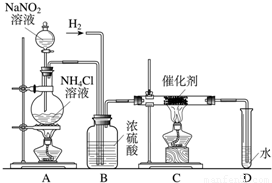
ŃĒĻõĖįÄĘŹĒŹµŃéŹŅ³£ÓƵďŌ¼Į£¬ŹµŃéŹŅŅ»°ćÓĆŃĒĻõĖįÄĘČÜŅŗÓėĀČ»Æļ§ČÜŅŗ·“Ó¦Ą“ÖĘČ”N
2£®N
2µÄ»ÆѧŠŌÖŹŹ®·ÖĪČ¶Ø£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀÄÜÓėH
2²æ·Ö»ÆŗĻÉś³ÉNH
3£®ĻĀĶ¼ĪŖÖĘȔɣĮæNH
3µÄ×°ÖĆ£ØÖĘČ”H
2µÄ×°ÖĆŅŃĀŌČ„£©£ŗ
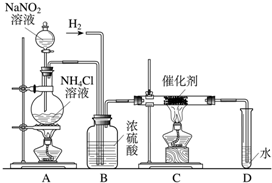
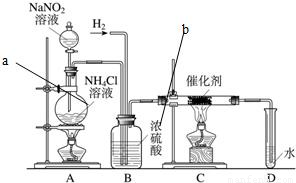
£Ø1£©C×°ÖƵÄÓ²ÖŹŹŌ¹ÜÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£®
£Ø2£©·“Ó¦Ź±N
2ŗĶH
2µÄ×ī¼ŃÖŹĮæ±ČŹĒ
14£ŗ3
14£ŗ3
£®Čē¹ū°““Ė±ČĄż½ųŠŠ·“Ó¦£¬·“Ó¦Ź±£¬DÖŠµ¼¹ÜæŚŹĒ·ń»įŅŻ³öĘųÅŻ£æ£ØŅŃÖŖNH
3¼«Ņ×ČÜÓŚĖ®£©£¬ĖµĆ÷ŅŻ³öĘųÅŻµÄŌŅņ£ŗ
ŅņĪŖŹĒ”°²æ·Ö»ÆŗĻ”±£¬ČŌ»įÓŠĪ“·“Ó¦µÄH2ŗĶN2ŅŻ³ö
ŅņĪŖŹĒ”°²æ·Ö»ÆŗĻ”±£¬ČŌ»įÓŠĪ“·“Ó¦µÄH2ŗĶN2ŅŻ³ö
£®
£Ø3£©BÖŠÅØĮņĖįµÄ×÷ÓĆŹĒ
øÉŌļ
øÉŌļ
£®
£Ø4£©ÓĆŹµŃé·½·ØÖ¤Ć÷Č·ŹµÓŠNH
3Éś³É£ŗ
ĻņDÖŠµĪČėĪŽÉ«·ÓĢŖČÜŅŗ£¬·ÓĢŖ±äŗģ
ĻņDÖŠµĪČėĪŽÉ«·ÓĢŖČÜŅŗ£¬·ÓĢŖ±äŗģ
£®




 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
 ŃĒĻõĖįÄĘŹĒŹµŃéŹŅ³£ÓƵďŌ¼Į£¬ŹµŃéŹŅŅ»°ćÓĆŃĒĻõĖįÄĘČÜŅŗÓėĀČ»Æļ§ČÜŅŗ·“Ó¦Ą“ÖĘČ”N2£®N2µÄ»ÆѧŠŌÖŹŹ®·ÖĪČ¶Ø£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀÄÜÓėH2²æ·Ö»ÆŗĻÉś³ÉNH3£®ĻĀĶ¼ĪŖÖĘȔɣĮæNH3µÄ×°ÖĆ£ØÖĘČ”H2µÄ×°ÖĆŅŃĀŌČ„£©£ŗ
ŃĒĻõĖįÄĘŹĒŹµŃéŹŅ³£ÓƵďŌ¼Į£¬ŹµŃéŹŅŅ»°ćÓĆŃĒĻõĖįÄĘČÜŅŗÓėĀČ»Æļ§ČÜŅŗ·“Ó¦Ą“ÖĘČ”N2£®N2µÄ»ÆѧŠŌÖŹŹ®·ÖĪČ¶Ø£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀÄÜÓėH2²æ·Ö»ÆŗĻÉś³ÉNH3£®ĻĀĶ¼ĪŖÖĘȔɣĮæNH3µÄ×°ÖĆ£ØÖĘČ”H2µÄ×°ÖĆŅŃĀŌČ„£©£ŗ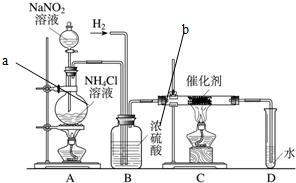 ŃĒĻõĖįÄĘŹĒŹµŃéŹŅ³£ÓƵďŌ¼Į£¬ŹµŃéŹŅŅ»°ćÓĆŃĒĻõĖįÄĘČÜŅŗÓėĀČ»Æļ§ČÜŅŗ·“Ó¦Ą“ÖĘČ”N2£®N2µÄ»ÆѧŠŌÖŹŹ®·ÖĪČ¶Ø£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀÄÜÓėH2²æ·Ö»ÆŗĻÉś³ÉNH3£®ĻĀĶ¼ĪŖÖĘȔɣĮæNH3µÄ×°ÖĆ£ØÖĘČ”H2µÄ×°ÖĆŅŃĀŌČ„£©£ŗ
ŃĒĻõĖįÄĘŹĒŹµŃéŹŅ³£ÓƵďŌ¼Į£¬ŹµŃéŹŅŅ»°ćÓĆŃĒĻõĖįÄĘČÜŅŗÓėĀČ»Æļ§ČÜŅŗ·“Ó¦Ą“ÖĘČ”N2£®N2µÄ»ÆѧŠŌÖŹŹ®·ÖĪČ¶Ø£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀÄÜÓėH2²æ·Ö»ÆŗĻÉś³ÉNH3£®ĻĀĶ¼ĪŖÖĘȔɣĮæNH3µÄ×°ÖĆ£ØÖĘČ”H2µÄ×°ÖĆŅŃĀŌČ„£©£ŗ