
| ����ϡ��������� | 18.25g | 36.5g | 54.75g | 73.0g | 100.0g |
| ������CO2������ | 1.1g | 2.2g | 3.3g | 4.4g | 4.4g |
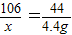
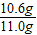 ×100%=96.4%
×100%=96.4%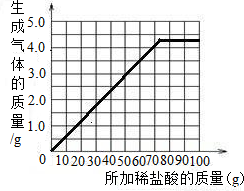


 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| XX���� ��Ҫ�ɷ֣�Na2CO3 Na2CO3������98% ���ã���ֽ����֯�� |
 |
| ����ϡ����Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ���������/g | 25 | 25 | 25 | 25 |
| �ձ�����ʣ���ʵ�����/g | 35 | 58 | 82.6 | 107.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ����ϡ��������� | 18.25g | 36.5g | 54.75g | 73.0g | 100.0g |
| ������CO2������ | 1.1g | 2.2g | 3.3g | 4.4g | 4.4g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij���������Ĵ����Ʒ�к����������ʣ�Ϊ����֤�ò�Ʒ��װ����̼���ơ�96%�����������־��ij����ѧϰС��ȡ11.0g�ô�����Ʒ������������Ϊ10%��ϡ���������вⶨ�����ʲ���ϡ���ᷴӦ�����й�ʵ���������£�
| ����ϡ��������� | 18.25g | 36.5g | 54.75g | 73.0g | 100.0g |
| ������CO2������ | 1.1g | 2.2g | 3.3g | 4.4g | 4.4g |
��ݴ˻ش��������⣺
��1��д��̼������ϡ���ᷴӦ�Ļ�ѧ����ʽ______��
��2�����11.0g����Ʒ�������Ĵ�̼���Ƶ�����Ϊ______ g���ɴ�֤���ò�ƷΪ______����ϸ��ϸ���Ʒ��
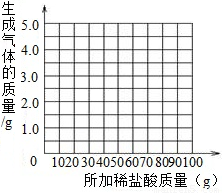
��3������ʵ�����ݣ�����ͼ�ϻ��Ƴ�����ϡ�������������ɵ�����������ϵ�����ߣ�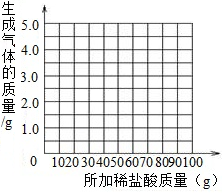
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�������п���ѧ�Ծ��������棩 ���ͣ������
| XX���� ��Ҫ�ɷ֣�Na2CO3 Na2CO3������98% ���ã���ֽ����֯�� |  |
| ����ϡ����Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ���������/g | 25 | 25 | 25 | 25 |
| �ձ�����ʣ���ʵ�����/g | 35 | 58 | 82.6 | 107.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com