
�����
b�����ɳ����Ļ�ѧ����ʽ____________________��
c����ˮ�ɻ��DZ����Ļ�ѧ����ʽ________________________��
 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
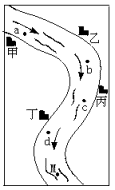
 29����ͼ��ʾ���Ӱ��߷ֲ��ס��ҡ�������������������������ԱΪ���ˮ�������ӱߣ��۲쵽��������b�����ֺ��ɫ���ǣ�c����ˮ�ɻ��DZ���壬d����ˮð���ݣ����a��ˮ��pH=12��M��ˮ��pH=5����֪�����������ŷŵķ�Һ�к����������еĸ�һ�֣�Na2CO3��FeCl3��HCl��Ba��OH��2���������������Ա�ƶϣ������ŷŵ���
29����ͼ��ʾ���Ӱ��߷ֲ��ס��ҡ�������������������������ԱΪ���ˮ�������ӱߣ��۲쵽��������b�����ֺ��ɫ���ǣ�c����ˮ�ɻ��DZ���壬d����ˮð���ݣ����a��ˮ��pH=12��M��ˮ��pH=5����֪�����������ŷŵķ�Һ�к����������еĸ�һ�֣�Na2CO3��FeCl3��HCl��Ba��OH��2���������������Ա�ƶϣ������ŷŵ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���½̲���ȫ��������꼶��ѧ��(�²�)��(�˽̰�) �˽̰� ���ͣ�022
��ͼ��ʾ���Ӱ��߷ֲ�������������������ԱΪ���ˮ�������ӱߣ��۲쵽��������b�����ֺ��ɫ���ǣ�c����ˮ�ɻ��DZ���壬d����ˮð���ݣ����a��ˮ����pH��12��m��ˮ����pH��5����֪ÿ�������ŷŵķ�Һ��ֻ�������������е�һ�֣�Na2CO3��FeCl3��HCl��Ba(OH)2��

(1)�������������Ա�Ʋⶡ���ŷŵ���________��m��һ�������е���������________��
(2)��д��b�����ɳ����ķ�Ӧ�Ļ�ѧ����ʽ��________��
(3)��д��c����ˮ�ɻ��DZ����ķ�Ӧ�Ļ�ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2002�������ʡ�п���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com