ŌŚĶس£×“æöĻĀ£¬ĀČĘųŹĒŅ»ÖÖ»ĘĀĢÉ«ĘųĢ壬ĆܶȱČæÕĘų“ó£¬Ņ×ČÜÓŚĖ®£®ŌŚ¹¤ŅµÉĻ”¢ŹµŃéŹŅÖŠæÉŅŌÖĘ×÷ĀČĘų£¬ÓĆĄ“ŃŠ¾æĀČĘųµÄŠŌÖŹ£¬²¢½«Ęä¹ć·ŗÓĆÓŚŹµ¼ŹÉś²śŗĶÉś»īÖŠ£®
£Ø1£©ŌŚ¹¤ŅµÉĻ£¬ÖĘČ”ĀČĘųµÄÖ÷ŅŖ¹¤ŅÕĮ÷³ĢČēĻĀ£ŗ

¢ŁĄūÓĆ·ē“µČÕɹæÉŅŌ“Óŗ£Ė®ÖŠĢįČ”“ÖŃĪ£¬øĆ¹ż³ĢÖ÷ŅŖ·¢Éś
ĪļĄķ
ĪļĄķ
£Ø”°ĪļĄķ”±»ņ”°»Æѧ”±£©±ä»Æ£®
¢ŚŅŖ³żČ„Ź³ŃĪĖ®ÖŠ»ģÓŠµÄÉŁĮæÄąÉ³£¬æÉŃ”ŌńµÄ²Ł×÷·½·ØŹĒ
¹żĀĖ
¹żĀĖ
£®
¢ŪĀČ»ÆÄĘČÜŅŗŌŚĶصēĢõ¼žĻĀ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
2NaCl+2H
2O
2NaOH+H
2ӟ+Cl
2ӟ
2NaCl+2H
2O
2NaOH+H
2ӟ+Cl
2ӟ
£®
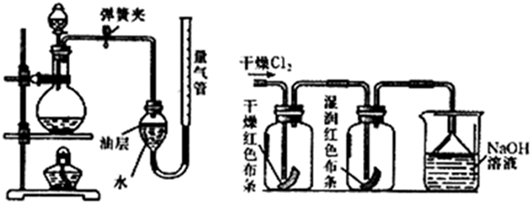
£Ø2£©ŌŚŹµŃéŹŅÖŠ£¬æÉŅŌÓĆ¼ÓČȶžŃõ»ÆĆĢŗĶÅØŃĪĖįĄ“ÖĘČ”ĀČĘų£¬æɲÉÓĆČēĶ¼×°ÖĆÖĘČ”ĀČĘų²¢²āĮæ²śÉśCl
2µÄĢå»ż£®
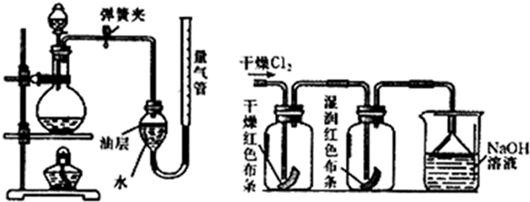
¢ŁÄܲÉÓĆøĆĘųĢå·¢Éś×°ÖƵĥķÓÉŹĒ
¶žŃõ»ÆĆĢŗĶÅØŃĪĖįÖĘČ”ĀČĘų£¬ŠčŅŖ¼ÓČČ
¶žŃõ»ÆĆĢŗĶÅØŃĪĖįÖĘČ”ĀČĘų£¬ŠčŅŖ¼ÓČČ
£®
¢ŚøĆ×°ÖĆÖŠÓĶ²ćµÄ×÷ÓĆŹĒ
·ĄÖ¹ĀČĘųÓėĖ®·“Ó¦
·ĄÖ¹ĀČĘųÓėĖ®·“Ó¦
£®
£Ø3£©ĀČĘųŌŚ³£ĪĀĻĀÄÜ·Ö±šÓėĖ®”¢¼īĄąµČĪļÖŹ·¢Éś·“Ó¦£¬²¢æÉŅŌ²śÉś¾ßӊɱ¾śĻū¶¾”¢ĘÆ°×£ØŹĒÓŠÉ«ĪļÖŹĶŹÉ«£©µČ×÷ÓƵÄĪļÖŹ£®
¢ŁĀČĘųÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCl
2+2NaOH=NaCl+NaClO+H
2O£®ĀČĘųÓėŹÆ»ŅČé·“Ó¦æÉÖĘĘÆ°×·Ū£¬ÓŠ¹Ų»Æѧ·½³ĢŹ½ĪŖ
2Cl2 +2Ca£ØOH£©2ØTCaCl2 +Ca£ØClO£©2 +2H2O
2Cl2 +2Ca£ØOH£©2ØTCaCl2 +Ca£ØClO£©2 +2H2O
£®
¢ŚĀČĘųŗĶĖ®·“Ó¦µÄŌĄķŹĒ£ŗCl
2+H
2O=HCl+HclO£®ĪŖĮĖÖ¤Ć÷°ŃŃõĘųĶØČėĖ®µÄ¹ż³ĢÖŠ£¬ĘšĘÆ°××÷ÓƵÄĪļÖŹŹĒ“ĪĀČĖį£Ø»ÆѧŹ½ĪŖHClO£©£¬Š”Ć÷Ķ¬Ń§½ųŠŠĮĖČēĶ¼ĖłŹ¾µÄĢ½¾æŹµŃ飮ÄćČĻĪŖŠ”Ć÷Éč¼ĘµÄ·½°øÄܹ»“ļµ½ÄæµÄĀš£æČōÄÜ£¬ĒėĖµĆ÷ĄķÓÉ£®Čō²»ÄÜ£¬Ēė²¹³äŹµŃé°ļÖśŠ”Ć÷“ļµ½ŹµŃéÄæµÄ£ØŠ“³öŹµŃé²½Öč”¢ĻÖĻó¼°½įĀŪ£©£®
£Ø4£©Ēė¼ĘĖć£ŗĪüŹÕ14.2gĀČĘų£¬ŠčŅŖĻūŗÄ20%µÄĒāŃõ»ÆÄĘČÜŅŗ¶ąÉŁæĖ£æ



 Ķ¬²½Į·Ļ°Ēæ»ÆĶŲÕ¹ĻµĮŠ“š°ø
Ķ¬²½Į·Ļ°Ēæ»ÆĶŲÕ¹ĻµĮŠ“š°ø




 £Ø2007?ĢØÖŻ£©ČēĶ¼ĖłŹ¾ĪŖŅ»Ģ׏µŃéŹŅÖĘČ”ĘųĢåµÄ×°ÖĆ£¬¼ÆĘųĘæÖŠµ¼¹ÜæŚĻĀ¶ĖÓ¦Éģµ½
£Ø2007?ĢØÖŻ£©ČēĶ¼ĖłŹ¾ĪŖŅ»Ģ׏µŃéŹŅÖĘČ”ĘųĢåµÄ×°ÖĆ£¬¼ÆĘųĘæÖŠµ¼¹ÜæŚĻĀ¶ĖÓ¦Éģµ½