
�±���NaOH��Ca(OH)2���ܽ������ ����ش��������⡣
����ش��������⡣

��1���ӱ������ݿ��Ի�õ���Ϣ��____ __��дһ������
��2����80��ʱNaOH�ı�����Һ������20�棬���Կ�����������_____________����ʱ��Һ��������������Ϊ ��������һλС����
 ��3��ij��ȤС��Բ��ֱ��ʵ��������ƹ�������ᴿ����������²������̣���ش�
��3��ij��ȤС��Բ��ֱ��ʵ��������ƹ�������ᴿ����������²������̣���ش�
��������м������Ca(OH)2��Ŀ����____ ��
�漰��Ӧ�Ļ�ѧ����ʽΪ__ ____��
������ҺB�е�������_______ _________��д��ѧʽ����
������������ľ�������Ǽ���Ũ���� �����ˡ�
������ʵ��ǰ�Ƶ���Ʒ������Ϊ10g��ʵ���Ƶá�����A���͡�NaOH���塱�������ֱ���5g��8.7g�������Ʒ�Ĵ���Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ҫ����գ�
(1)���ʯ��ʯī�������ʲ���ܴ��ԭ���� ��
(2)������̼��һ����̼���ʲ���ܴ��ԭ����
(3)̼ԭ�Ӻ�þԭ�ӵı��ʲ�ͬ��
��4��ˮ������������һ�ֽ������׳ƣ����ֽ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�Ǽס��������ʣ��������ᾧˮ�����ܽ����ߣ��ݴ˻ش��������⣺
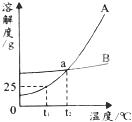
��1��t1��ʱ��A���ʵ��ܽ��Ϊ g��
��2��a��ĺ����� ����t2��ʱ��A��B�����ʵ��ܽ����ͬ
��3���ܽ�����¶�Ӱ��С�������� ��
��4����A��B�Ļ�����з���A���ʣ�һ����� �ķ�����
��1��t1��ʱ��A���ʵ��ܽ��Ϊ g��
��2��a��ĺ����� ����t2��ʱ��A��B�����ʵ��ܽ����ͬ
��3���ܽ�����¶�Ӱ��С�������� ��
��4����A��B�Ļ�����з���A���ʣ�һ����� �ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ֻ�������ʹ�õ�﮵���ڷŵ�ʱ�����ķ�Ӧ�ɱ�ʾΪLi+MnO2==LiMnO2������˵����ȷ����
A����Ӧ��MnO2�Ǵ��� B���÷�Ӧ�����û���Ӧ
C����Ӧ��Ҫͨ����ܽ��� D����Ӧ�л�ѧ ��ת���ɵ���
��ת���ɵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������ܹ��ﵽʵ��Ŀ�ĵ���
A���ڿ��������ռ����ͭ�ͻƽ�
B����Ʒ���ķ�������ʳ���κ�ҵ����
C���������ȥ�Ȼ�����Һ�е���������
D���÷�̪��Һ��������������Һ�Ƿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������������仯���͵��ǣ�������
A.ˮ����ˮ����ˮ��
B. �������ڴ�����������ˮ��
C.ţ�̱���
D. ʯī��һ��������ת��Ϊ���ʯ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ͭ��ϡ���ᡢ����������Һ������ͭ��Һ�������ʣ�����������ͼ��ʾ�Ĺ�ϵ���磨ͼ�еġ�������ʾ���������������ܷ�����ѧ��Ӧ���������й�˵������ȷ���ǣ� ��
A�������ʿ���Ϊ����������Һ
B��������һ��������ͭ
C�����Ͷ��ķ�Ӧһ�����û���Ӧ
D�������ʿ���ʹ��ɫʯ����Һ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʵ������ȡ������̼�����������ʵ�װ�úͲ���ʾ��ͼ��
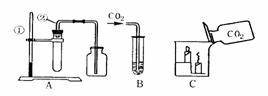
�Իش������й����⣺
(1)д�������ٺ͢ڵ����ƣ����� ������ ��
(2)A����ȡ������̼�Ļ�ѧ����ʽ�� ��
(3)Ϊ�˼���Aװ�õļ���ƿ���Ƿ����������̼������ȼ�ŵĻ����� ����ʵ�飬�������Ϩ��֤���Ѿ�������
(4)��B���Թ���ʢ������ɫʯ����Һ���۲쵽��������
�����Թ���ʢ���dz���ʯ��ˮ���۲쵽��������
��
(5)C��ʾ��ʵ��˵���˶�����̼������Щ���ʣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com