
 =9.8%��
=9.8%��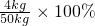 =8%��
=8%�� �����㼴�ɣ�
�����㼴�ɣ� �����㼴�ɣ�
�����㼴�ɣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
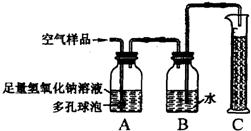 22���ݱ�������2005��1��1���𣬹���ʡ���ػ������Ž���ʵ��������Ⱦʵʩ�ϸ�Ļ�����ܣ�����ѧУ��ѧʵ����Ҫ�ŷųɷָ��ӵ���Ⱦ�����Ҳ����Ϊ������ܷ�Χ��ijУ��ѧ��ȤС���ͬѧ�ڼ�ʵ�����н����������ֱ���̼��������Ӧ��ʵ���Ϊ�˽��ʵ�����������Կ����ɷ���ɵ�Ӱ�죬�������������ʵ��װ�ý���ʵ�飨ͼ�ж�����ݵ������ǣ�������������Һ�ĽӴ������ʹ��Ӧ��ֽ��У���
22���ݱ�������2005��1��1���𣬹���ʡ���ػ������Ž���ʵ��������Ⱦʵʩ�ϸ�Ļ�����ܣ�����ѧУ��ѧʵ����Ҫ�ŷųɷָ��ӵ���Ⱦ�����Ҳ����Ϊ������ܷ�Χ��ijУ��ѧ��ȤС���ͬѧ�ڼ�ʵ�����н����������ֱ���̼��������Ӧ��ʵ���Ϊ�˽��ʵ�����������Կ����ɷ���ɵ�Ӱ�죬�������������ʵ��װ�ý���ʵ�飨ͼ�ж�����ݵ������ǣ�������������Һ�ĽӴ������ʹ��Ӧ��ֽ��У����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
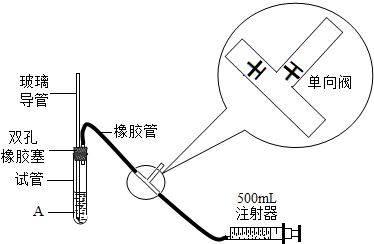
| S02���Ũ����ֵ����λmg?m-3�� | ||
| һ���� | ������ | ������ |
| 0.15 | 0.50 | 0.70 |
| �� �� | ��һС�飨ʵ���ң� | �ڶ�С�飨���ң� |
| �������� | 110 | 150 |
| ������S02�ĺ�������λ��mg?m-3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
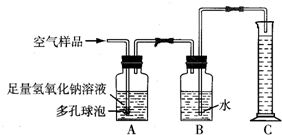 A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ���
A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com