
��ȡNaCl��BaCl2�Ĺ�������32.5 g������100 g����ˮ����ȫ�ܽ����û����Һ����μ�����������Ϊ10%��Na2SO4��Һ����Ӧ����BaSO4�������������������Na2SO4��Һ��������ϵ����ͼ��ʾ���Իش��������⣺
(��ʾ��BaCl2+Na2SO4====BaSO4��+2NaCl)

(1)��ȫ��Ӧ������BaSO4����______________g��
(2)ǡ����ȫ��Ӧʱ����Na2SO4��Һ��������____________________g��
(3)ǡ����ȫ��Ӧʱ������Һ�����ʵ����������Ƕ��٣�(��ȷ��0.1%)
���⿼����ͼ����л�ѧ����ʽ�����������
(1)������ͼʾ��֪����ȫ��Ӧ������BaSO4����������Ϊ23.3 g��
(2)����BaSO4����������������Ƶ�������������������Һ�����ʵ������������������������Һ��������
(3)ǡ����ȫ��Ӧ����Һ���������Ȼ��ƣ����ݳ���������������÷�Ӧ���ɵ��Ȼ��Ƶ�����������ԭ�������Ȼ��Ƶ�������Ϊ��Ӧ����Һ�����ʵ���������һ�������ǡ����ȫ��Ӧʱ������Һ�����ʵ�����������
�𰸣�(1)23.3��(2)142
(3)�⣺��BaCl2������Ϊx����Ӧ���ɵ�NaCl������Ϊy��
BaCl2+Na2SO4====BaSO4��+2NaCl
208 233 117
x 23.3 g y
 =
= =
=
x=20.8 g��y=11.7 g
ǡ����ȫ��Ӧʱ����Һ��NaCl������Ϊ
11.7 g+(32.5 g-20.8 g)=23.4 g
NaCl��Һ���ʵ���������Ϊ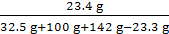 ��100%=9.3%
��100%=9.3%
��(��)


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���˵�θҺ��,����(HCl)������������Լ��0.45%��0.6%��������������������:�ٴٽ�θ����ø�Ĵ�����,ʹ������ˮ�������������;��ʹ�������ʽ�һ��ˮ��;��ɱ����
�����������ݻش���������:
(1)�����Al(OH)3�Ļ�ϼ�����θ�����,������Ӧ�Ļ�ѧ����ʽΪ��____��
(2)����ʳ��������NaCl����������������
A.�ٽ���������������� B.������ ��������ʳ��
��������ʳ��
C.����θ����� D.����ȱ���Լ�״���״�
(3)���ࡢ������������֬��ά���ء����κ�ˮ�����������Ӫ�����ʡ���������Ӫ��������,����������,�Ϳ�������������ֱ�ӱ��������յ�����_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ҵ�����⽨һ���᳧������������Ĺ����л����SO2����SO2ֱ��������ŷţ������γ����꣬�����pH<________��Ϊ��ֹSO2��Ⱦ�����������·�������Ϊ���е���________(�����)��
�ٽ�����̴ѣ���������߿��ŷ�
���÷�NaOH��Һ����SO2
������ʯ������SO2
���÷�������Һ����SO2
������д�����ܷ�����һ����ѧ����ʽ��_______________________________
_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ��X��Һ��AgNO3��Na2CO3��Na2SO4������Һ������Ӧ�����ɰ�ɫ��������X�����������������ʵ���Һ(����)

A.HNO3��KNO3�������� B.HCl��H2SO4
C.BaCl2��CaCl2 D.NaOH��Ca(OH)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�Ϳ�������Ҫ����Ȼ��Դ���Ӻ�ˮ�Ϳ����з�������ֳɷֿɸ��õķ������ࡣ
(1)ijС����ƽ��������ξ����ס��ҡ�������װ�ã����շ�����ϴ���N2��

���п��ܷ�����Ӧ�Ļ�ѧ����ʽΪ__________������Ũ�����������________________�����п��Լ�����Լ�Ϊ__________��
(2)��ˮ�и���þԪ�أ���ͼ�ǴӺ�ˮ����ȡþ������ͼ������ͼ��A��B�Ļ�ѧʽ����Ϊ_____________��______________��

(3)��ˮ������ɹ�õ����κ�ʳ�εı�����Һ����һƿʳ�α�����Һ�м���һ������ʳ�ξ��壬�����¶Ⱥ�ˮ�����������䣬һ��ʱ���ʳ�ξ��������______(����䡱�仯������ͬ)��ʳ�ξ������״________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���м�ͥ�е��������ܷ�ֹ�˵��������(����)
A.�˵�ʹ�������ˮϴ����������
B.���ò���ʱ�˵�����Ϳһ��ֲ����
C.�Ѳ˵�����ڳ�ʪ�ĵط�
D.�ò���ֲ˵�������ͨ�˵�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ����˵��Zn��Ag���õ���(����)
A.Zn����AgNO3��Һ��Ӧ�û���Ag
B.Zn��ϡ���ᷴӦ��Ag����
C.��Ȼ����û���Ե�����ʽ���ڵ�Zn�������Ե�����ʽ���ڵ�Ag
D.Zn���۵�Ϊ420�棬Ag���۵�Ϊ962��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���������кͷ�Ӧ����(����)
A.�����ữ�������ʯ�Ҹ���
B.θ����ڹ���IJ�����ҽ�����ú�������������ҩ�����к���θ��
C.�ó涣ҧ�˵�Ƥ�����ڳ����ᣬ���Ϳ���������ʵ�ҩˮ�Ϳɼ���ʹ��
D.����������ʴ����ϡ���������ϴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��������Ba(OH)2��Һ��Na2CO3��Һ���ǡ����ȫ��Ӧ����Ӧ��Ļ�����м���ϡ���ᣬ�����������������ϡ���������Ĺ�ϵ������ͼ��ʾ������˵������ȷ���ǣ� ��

A��N ��ʱ��������Һ��pH=7
B��Q��ʱ��������Һ�е�����ֻ����BaCl2
C��O��P�η�����Ӧ�Ļ�ѧ����ʽΪNaOH+HCl=NaCl+H2O
D��P��Q�η�����Ӧ�Ļ�ѧ����ʽΪBa(OH)2+2HCl=BaCl2+2H2O
| M������/ g | S������/ | M2S������/ g | |
| �� | 6.0 | 2.5 | 7.5 |
| �� | 7.0 | 1.5 | 7.5 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com