
���� ��1�����ݻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ������з�����
��2�����������غ㶨�ɽ��л�ѧ����ʽ����ƽ��
��3������A�г��ֺ�ɫ����˵����һ����̼�������ų�����ڽ��з�����
��4���������ֻ��������һ����̼����Ҳ��������������з�����
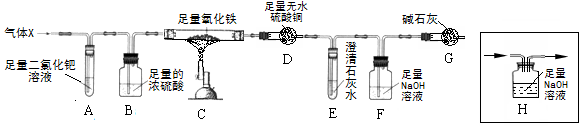
��5����������������װ�ý�����ƣ�
��6������װ��D��������m1gΪˮ��������װ��E��F�������ӵ��ܺ�Ϊm2gΪ������̼���������н��
��� �⣺��1����ѧ��Ӧǰ��ԭ�ӵ��������Ŀ���䣬��CO+PdCl2+H2O=CO2+Pd��+2X��֪����Ӧ������1��̼ԭ�ӣ�2����ԭ�ӣ�1����ԭ�ӣ�2����ԭ�ӣ�2����ԭ�ӣ����Ը÷�Ӧ��X�Ļ�ѧʽ��HCl��
��2���������غ㶨�ɻ�ѧ��Ӧǰ��ԭ�ӵ��������Ŀ�����֪��4Fe2O3+nCH4$\frac{\underline{\;����\;}}{\;}$8Fe+nCO2+2nH2O��n��ֵΪ3��
��3��A�г��ֺ�ɫ���壬�����������ʣ�����˵����һ����̼�������ų�����ڣ�
��4�����ֻ��������һ����̼���壬Ҳ���Գ����������������ҵĽ��۲���ȷ��
��5������������֪��Ҫ��֤ʣ��ļ����Ƿ��������Ҫ��A��B֮������װ��H������Hװ����ͼ�� ��
��
��6����4Fe2O3+3CH4$\frac{\underline{\;����\;}}{\;}$8Fe+3CO2+6H2O��֪������ˮ�Ͷ�����̼������Ϊ����6��18������3��44��=9��11������m1��m2=9��11ʱ��������ۡ�������
�ʴ�Ϊ����1��HCl��
��2��3��
��3���ڣ�
��4������ȷ��
��5��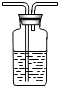 ��
��
��6��9��11��
���� �������ճ�����������ʣ��������������ʶ������м��顢�����������DZ������յĻ������ܣ�Ҳ�ǻ�ѧʵ�鿼����ȵ�֮һ��


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | A | B | C | D |
| ��Ӧǰ����/g | 2 | 26.5 | 2 | 10 |
| ��Ӧ������/g | 16.9 | 2 | 2 | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
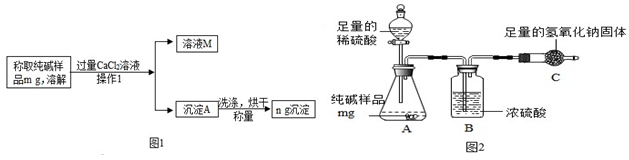
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������ | |
| B�� | �þ�����ϩ���������������ľ�Ե�� | |
| C�� | ����ʯ����ʳƷ����� | |
| D�� | ���¼Ʋ���ˮ��ָʾ�¶ȱ仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��ԭ�ӵĺ�����ӷ�5���Ų� | B�� | ��Ԫ�ص�ԭ������Ϊ38 | ||
| C�� | �����ӵĻ�ѧ����ΪSr2+ | D�� | �����ȵĻ�ѧ���ʲ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CaO+H2O�TCa��OH��2 | B�� | Zn+H2SO4�TZnSO4+H2�� | ||
| C�� | 2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2�� | D�� | Na2CO3+Ca��OH��2�TCaCO3��+2NaOH |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com