
12��)��ͼ��̼���仯�����ת����ϵͼ��
��ش��������⣺
��1�������ں���̼Ԫ�����ʵĻ�ѧʽ�� ��
��2��ת����ϵͼ����CO2��CaCO3�ķ�Ӧ��ѧ����ʽ�� ��
��3������þ��������������ȼ�գ�Ҳ�����ڶ�����̼��ȼ����������þ(MgO)��̼���ʣ�þ�ڶ�����̼��ȼ�յĻ�ѧ����ʽΪ�� ��
�ɴ����ȼ�ա������ʲô�µ���ʶ�� ����һ��ɣ���
��4�������й�̼���仯�����˵����ȷ���� ��
A��������̼�ǵ����������Ҫ����
B����̼ȼ�ϲ���ȫȼ�ջ�����һ����̼��һ����̼�ж�
C��Һ̬������̼�������Ϊ����ʱ���ȣ������˿�ȼ����Ż��
��1��CO ��2��CO2+Ca(OH)2===CaCO3��+H2O
(3)2Mg+2CO2��ȼ2MgO+C ȼ�ղ�һ����Ҫ���� �����þ��ȼ�ղ�����CO2���
(4)B
���������������1������ת����ϵͼ���������Կ�������һ��̼���ǡ����������ں���̼Ԫ�����ʵĻ�ѧʽ��CO ��2��ת����ϵͼ����CO2��CaCO3�ķ�Ӧ��ѧ����ʽ��CO2+Ca(OH)2===CaCO3��+H2O
��3�����ݽ���þ�ڶ�����̼��ȼ����������þ(MgO)��̼���ʣ���ѧ����ʽΪ��2Mg+2CO2��ȼ2MgO+C �ɴ˶�ȼ�ա�����µ���ʶ��ȼ�ղ�һ����Ҫ���� �����þ��ȼ�ղ�����CO2���
��4��A�������������Ҫ�����Ƕ�������������������Ƕ�����̼������B����̼ȼ�ϲ���ȫȼ�ջ�����һ����̼��һ����̼�ж�����ȷ��C��Һ̬������̼�������Ϊ��������ʱ���ȣ��������¶ȣ�ʹ�¶ȵ��ڿ�ȼ����Ż�㣬���Ҹ�������
���㣺̼���仯����Ļ�ѧ���ʣ�ȼ�պ����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
2014�����ӡ�3��15���ᡰ��������Ԥ��������Ϳ��Һ��һЩ�к��ɷ����س��ꡣij��Ʒ������Һ��װ��ǩ�ϵIJ���������ͼ������ϸ�Ķ����۲죬���ݴ�ͼ�������Ʋ�����Һ�����ʣ������һ�㣺
��1���������ʣ� ��
��2����ѧ���ʣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��8�֣�������������ת����ϵͼ����ɫ����B�ڷ�Ӧ�١����������ͻ�ѧ���ʶ�û�иı䣬J��ʹ��ɫʯ����Һ��죬J����Ҳ���ȶ����ֽ���������I��G����ݴ˷������ش��������⣺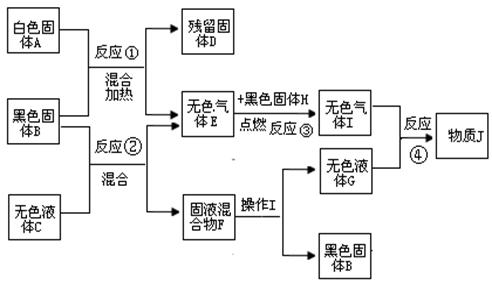
��1��д�����з�Ӧ�����ֱ���ʽ����Ӧ�� ��
��Ӧ�� �� ��Ӧ�� ��
��2������������ƽ� ��
��3����������D���ܽ⡢���ˡ� ����ɺ�ɻ������B������B�ڷ�Ӧ�١������� ���ã����ܽ⡢�����ж�Ҫ�õ��������������÷ֱ��ǣ�
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��һ����ɫ���壬�������� ��
�� ��
�� �е�һ�ֻ��ֻ����ɵģ��ô˻��������������ʵ�飺
�е�һ�ֻ��ֻ����ɵģ��ô˻��������������ʵ�飺
��1����������ͨ�����ʯ��ˮ�У���������
��2���������徭���쵼�ܵ����������ȼ�գ��ڻ����Ϸ���һ���������ձ����ձ��ڱ�����ˮ����֡�
��������ʵ�������ƶϣ�
��������һ������______��������_______�����ܺ���______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���Ϣ������
��12�֣�����ˮ��ͷ����ʹ���������⣬С�����Լ�ˮ��ͷ��ȡ��һЩ������Ʒ����ͼ����ʾ��װ�ý���ʵ�顣
��1��B������ʯ��ˮ����ǣ���Ӧ����ʽΪ ��C���ƾ��Ƶ�������
��2��ʵ�������С�������õĺ�ɫ�������ʷ���������ϡ�����У�����û�����ݣ���˵���������� ���л�û�У�����
Ϊ�����������С�������������ϣ�
����֪������������FeO��Fe3O4��Fe2O3������һ�������£�������ʧȥ���е��������ձ���ԭΪ����
��ij����������������һ����̼�����ȷ�Ӧ���������������ݲ����Ƴ���ͼ��
ͨ����������ȷ����
��700��ʱ��������һ����̼���з�Ӧ�IJ����� ����FeO��Fe3O4��Fe��.
��С��ʵ��ʧ�ܵ���Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ӧ�÷dz��㷺����������ͭ����������ϢϢ��ء�
��1�������г��õ��������ˣ���Ҫ������������ ��
A�������� B�������� C����չ�� D���н�������
��2��Ŀǰ����������50%���ϵķϸ����õ��������ã���Ŀ���� ��
A����Լ������Դ B���������ɿ��� C����ֹ�������⣮
��3�����������õĿ���ʴ�ԣ����������ڿ�������������Ӧ�������������һ�����ܵ���������Ĥ���Ӷ���ֹ����һ������������������Ӧ�Ļ�ѧ����ʽ ��
��4���������Լ���֤�����ֽ����Ļ��˳���ܴﵽĿ���� ������ţ���
A����������Һ B������������Һ C������ͭ��Һ
��5����������ᶼ�ܳ����⣬д��������������Ҫ�ɷַ�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��11�֣���13�֣�
����B��һ�ֳ�������;�㷺�Ľ�����BԪ���ڵؿ��к���������������֪����B�ܷ�������һϵ�б仯�����ƶϣ�
��1��д��A��E�����ƣ�A_______ B._____ C.______ D.______ E.________��
��2��д���١��ܸ�����Ӧ�Ļ�ѧ����ʽ��
��___________����_______��_____��_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
N(NO2)3�ǿ�ѧ��2011�귢�ֵ�һ�����ͻ��ȼ��.�Լ���
��1��N(NO2)3��Nԭ������ԭ�ӵĸ�����
��2��N(NO2)3������Է���������д����Ҫ�������,��ͬ��
��3��N(NO2)3��N����������
��4��15.2g��NԪ�ص�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com