

| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ������人������ѧ�������棩 ���ͣ�̽����
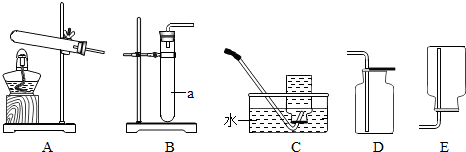
ij��ѧС��ɹ��������ͼ��ʾʵ�飨װ�����������ã�����֤�˶�����̼����ɡ�
̽���������£�

1. ����װ�и����״ľ̿���Թ�D������Ϊ50.7g��װ�м�ʯ�ҵ�װ��C����Ϊ112.3g������A��B��Dװ�ã�
2. �ӳ���©����������3%�Ĺ���������Һ������Cװ�ã���ȼ�ƾ��ƣ�
3.��D�з�����Ӧ��Ϩ��ƾ��ƣ���ȴ��
4. �����Թ�D��ʣ����������Ϊ50.1g��װ��C������Ϊ114.5g
��֪����ʯ�ҵijɷ��������ƺ��������ƣ�ľ̿�е����ʲ����뷴Ӧ��B��C����װҩƷ����������ȫ����������ʡ���ش��������⣺
װ��A��Ӧ�Ļ�ѧ����ʽΪ_____________________���÷�Ӧ����_________��Ӧ
�ƾ��Ƽ��ȵ�������_____________________
Ϊ��С���������ȴ��������Ҫע���������_______________________
����ʵ�����ݼ���μӷ�Ӧ������������Ϊ����ʽ�����㣩_____________���Ӷ������������̼��̼����Ԫ�ص������ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0101 �¿��� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧС��ɹ��������ͼ��ʾʵ�飨װ�����������ã�����֤�˶�����̼����ɡ�

̽���������£�
I������װ�и����״ľ̿���Թ�D������Ϊ50.7 g��װ�м�ʯ�ҵ�װ��C����Ϊl12.3 g������A��B��Dװ�ã�
II���ӳ���©����������3%�Ĺ���������Һ������Cװ�ã���ȼ�ƾ��ƣ�
��D�з�����Ӧ��Ϩ��ƾ��ƣ���ȴ��
���������Թ�D��ʣ����������Ϊ50.1 g��װ��C������Ϊ114.5 g
��֪����ʯ�ҵijɷ��������ƺ��������ƣ�������ʾ�������������������̼������ѧ��Ӧ����ľ̿�е����ʲ����뷴Ӧ��B��C����װҩƷ����������ȫ����������ʡ���ش��������⣺
(1)װ��A��Ӧ�Ļ�ѧ����ʽΪ_______________________________���÷�Ӧ����________��Ӧ��
(2)װ��B��������_________________��װ��D�����м�ס�Թܵ����ϲ���ԭ����_______________���ƾ��Ƽ��ȵ�������_________________��
(3)Ϊ��С���������ȴ��������Ҫע���������____��
(4)����ʵ�����ݼ���μӷ�Ӧ������������Ϊ����ʽ�����㣩_______________________________���Ӷ������������̼��̼����Ԫ�ص������ȡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com