
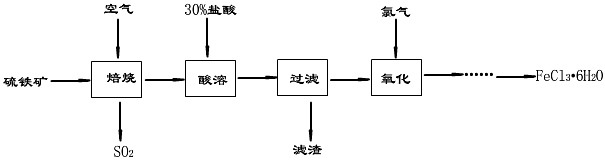
 2NaOH+ Cl2��+ H2��������Ҫ71t������������ˮ��������������Ҫ������10%�Ĵ��ζ��ٶ֣�ͬʱ�����ռ���ٶ֣�
2NaOH+ Cl2��+ H2��������Ҫ71t������������ˮ��������������Ҫ������10%�Ĵ��ζ��ٶ֣�ͬʱ�����ռ���ٶ֣� ���Σ�ϣȣ����������ã�����
���Σ�ϣȣ����������ã�����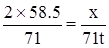 ���������������������������֣�
���������������������������֣� ���������������������������֣�
���������������������������֣�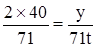 �����������������������������֣�
�����������������������������֣�

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
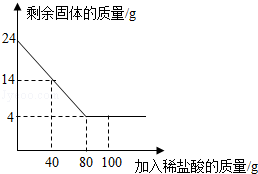
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| | �������� | �������� |
| ά����C ��ѧʽ��C6H806 | ��ɫ���壬������ˮ����������Һ���ȶ������Ի������Һ���ױ������������� | �ٽ�����������������ǿ����Լ����ĵֿ��������� |
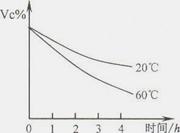
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ��ϡ�����������g�� | 10 | 10 | 10 | 10 |
| ʣ�����������g�� | 9.10 | 8.45 | 7.80 | 7.80 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com