
���Թ��У�������ͼ����װ�ûش����⣨װ��B������R�Ƿ�Һ©�������Ե��ڻ�������Һ��ĵμ��ٶȣ���
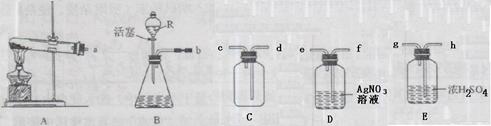 |
��1����������أ��ö�����������������ȡ����Ӧѡ�õķ���װ���� ���仯ѧ����ʽΪ ��
��2��ʵ������п��ϡ���ᷴӦ����ȡ��������Ӧѡ�õķ���װ���� ���仯ѧ����ʽΪ ��
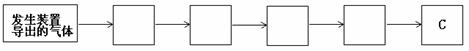
��3���ã�2�����ַ����Ƶõ�����������������HCl�����ˮ������װ��D��E�зֱ�ʢ����������Һ��Ũ���ᣬ�����ռ�һƿ������������������ӷ���װ�õ�����������
 |
��4����װ��B��������ȡ�����������������һ�������� ��
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com