
| �������ʵ����� | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
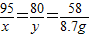
 =75g����������ʵ�������õ�������������Һ��������������Ϊ��
=75g����������ʵ�������õ�������������Һ��������������Ϊ��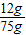 ×100%=16%��
×100%=16%��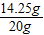 ×100%=71.25%
×100%=71.25%

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������ʵ����� | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������ʵ����� | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� �� ���ʵ����� |
1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �������ʵ����� | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���������Ȼ�þ�������ƵĹ���������Ʒ��С��ͬѧ��ⶨ��Ʒ���Ȼ�þ�������������ȳ�ȡ�û������Ʒ20g����ȫ����ˮ�У�Ȼ��ȡ����һ��������������������������Һ100gƽ�����Ĵμ������У������ʵ���������ݼ��±�����������������йؼ��㣺
|
| 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��1���ϱ���X����ֵΪ_________��
��2������ԭ����������Ʒ���Ȼ�þ�����������Ƕ��٣�����������ػ�ѧ����ʽ���м��㣬д����Ҫ�Ĺ��̣�
��3������ʵ�������õ�������������Һ����С��ͬѧ����ʵ���������е�80g������������Ϊ30%������������Һ�����Ƶģ�ͨ�������֪����� gˮ���������ʵ��������������������������������Һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com