
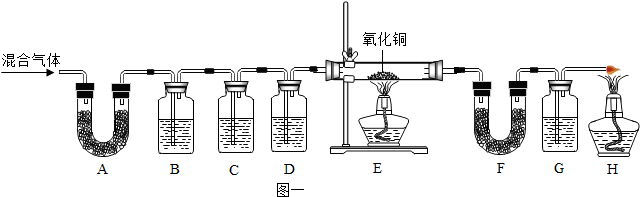
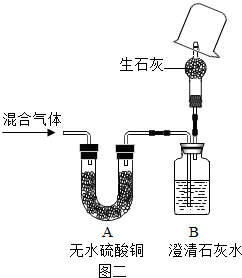
| ||
| ||
| 16 |
| 64+16 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ڿ�����ȼ�շ�������ɫ���� |
| B������ɫʯ����ҺȾ�ɵ�С�����������CO2�Ӵ���ֽ����� |
| C��þȼ��ʱ�ᷢ��ҫ�۵İ� |
| D���Ѵ��л��ǵ�ľ������ʢ�������ļ���ƿ�У�ľ����ȼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | �Ҵ� | ���� | ������̼ | ˮ | X |
| ��Ӧǰ����g | 4.6 | 8 | 0 | 0 | 0 |
| ��Ӧ������g | 0 | 0 | 4.4 | 5.4 | m |
| A������m��ֵΪ2.8 |
| B��X�����Ǹ÷�Ӧ�Ĵ��� |
| C�����������������Լ���X������ |
| D������X���ܺ���̼Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ƻ���к���Cu2+ |
| B��ƻ���е�Fe2+���Fe3+ |
| C��ƻ���к���Na+ |
| D��ƻ���к���OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com