
| ���ʵ����� | ƽ��ֵ |
| ��Ӧǰ���ձ�+���� | 222.0g |
| ʯ��ʯ��Ʒ | 10.0g |
| ��Ӧ���ձ�+��Һ | 226.2g |
���� ��1��������Ԫ����ɣ�һ��Ԫ������Ԫ�صĻ������������������ָ���������������ȫ���������ӵĻ��������ָ�������������ȫ�������������ӵĻ��������ָ������������Ӻ�������ӵĻ����
��2�����������������Һ�������仯�����⣮
��� �⣺��1��̼��ƶ����ɽ������Ӻ����������ɵĻ�����������Σ������
��2����̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2�� ��������
100 44 100-44=56
x 226.2g-222.0g=4.2g
$\frac{100}{x}$=$\frac{56}{4.2g}$
x=7.5g
��ʯ��ʯ��Ʒ��̼��Ƶ���������$\frac{7.5g}{10g}$��100%=75.0%
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ���������75.0%��
���� �����ǶԻ�ѧ����ʽ������ۺϿ����⣬���������غ㶨�ɣ�������Һ�������仯�ǽ���Ĺؼ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʱ���Ż��������Ϲ��� | |
| B�� | �������õĵؽ�ǰ�Ƚ��еƻ�ʵ�� | |
| C�� | �ü�ȩ��Һ����ʳ�ú���Ʒ�Ա��� | |
| D�� | �����ղ�����ζ�ķ���������֯��ʹ�ë֯�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������һ���������� | |
| B�� | �����ʵĻ�ѧʽΪMgCO3 | |
| C�� | ��������þ����Ԫ�ص�������Ϊ 1��1 | |
| D�� | ����������Ԫ�ص�����������25% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
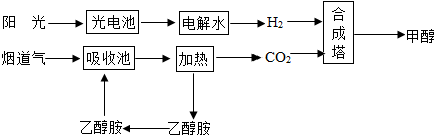
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
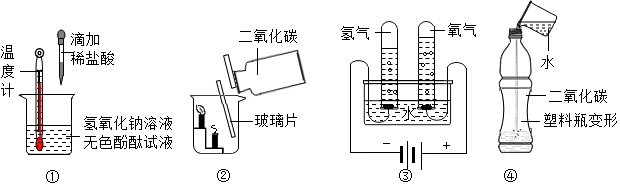
| A�� | �٢ۢ� | B�� | �ڢۢ� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
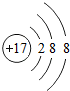 ��ʾ����Cl-��
��ʾ����Cl-��| ���� | A | B | C | D |
| ��Ӧǰ������/g | 6.4 | 3.2 | 4.0 | 2.8 |
| ��Ӧ�������/g | 5.2 | ���� | 7.2 | 2.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | þ | B�� | ͭ | C�� | ̼ | D�� | п |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com