
| ʵ���� | ʵ����� | ʵ������ |
�� |
�ֱ����Թ�A��B�м��� 5mL 5%����ҺŨ�ȣ� H2O2��Һ��������2 ����ͬŨ�ȵ�CuSO4��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݣ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5mL 5%H2O2��Һ��5mL 10%H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
| ||
| ||

| ||
| ||


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5mL 5% H2O2��Һ��������2�ε�Ũ�� FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݣ� | �Թ�A�в��ٲ������ݣ��Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5mL 5% H2O2��Һ��5mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ�п�ģ�⻯ѧ�Ծ��������棩 ���ͣ�̽����
Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش�
|
��� |
���� |
ʵ������ |
|
�� |
�ֱ����Թ�A��B�м���5mL 5% H2O2��Һ��������2�ε�Ũ�� FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݣ� |
�Թ�A�в��ٲ������ݣ��Թ�B�в��������������� |
|
�� |
��ȡ��֧�Թֱܷ����5mL 5% H2O2��Һ��5mL 10% H2O2��Һ |
�Թ�A��B�о�δ���Լ��������ݲ����� |
��1����������ֽ�Ļ�ѧ����ʽΪ ��
��2��ʵ��ٵ�Ŀ���� ��
ʵ���еμ�FeCl3��Һ��Ŀ���� ��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������ ������ʵ�����ṩ���Լ�����
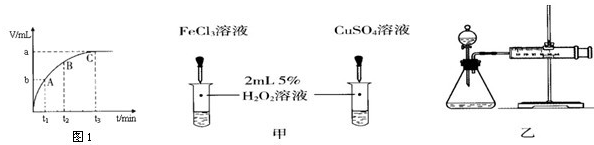
��4��ijͬѧ��50mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų����������뷴Ӧʱ��Ĺ�ϵ��ͼ1��ʾ����A��B��C��������ʾ��˲ʱ��Ӧ������������ ��
��5�������ǻ�ѧ��Ӧǰ�� ��δ�ı��һ�ѧ���ʣ�
��6������H2O2�ֽⷴӦ��Cu2+Ҳ��һ���Ĵ����ã�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�飮��ش�������⣺

�ٶ��Է�������ͼ��ͨ���۲� �����ԱȽϵó����ۣ���ͬѧ�����FeCl3��Ϊ ��Ϊ�������������� ��
�ڶ�����������ͼ����ʾװ��������ʵ�飬ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�ʵ������Ҫ������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�걱���з�ɽ�����꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�̽����
��8�֣�Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�顣
|
ʵ���� |
ʵ����� |
ʵ������ |
|
�� |
�ֱ����Թ�A��B�м��� 5 mL 5��(��ҺŨ��) H2O2��Һ��������2 ����ͬŨ�ȵ�CuSO4��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݡ� |
�Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
|
�� |
��ȡ��֧�Թֱܷ����5mL 5��H2O2��Һ��5 mL 10��H2O2��Һ |
�Թ�A��B�о�δ���Լ��������ݲ����� |
��1����������ֽ�Ļ�ѧ����ʽΪ ��
��2��ʵ��ٵ�Ŀ���� ��
ʵ���еμ�CuSO4��Һ��Ŀ���� ��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�����Ƶ�ʵ�鷽���� ������ʵ�������ṩ�ļ����Լ�����
��4������H2O2�ֽⷴӦ��Fe2(SO4)3��ҺҲ��һ���Ĵ����á�Ϊ�Ƚ�Fe2(SO4)3��CuSO4��Һ��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣��ش�������⣺

��ͼ��ʾ��ͬѧ�Ƿֱ�Ӷ��ԺͶ����ǶȽ����˱Ƚϡ�
�ٶ��Է�������ͼ��ͨ���۲� �����ԱȽϵó����ۡ�
�ڶ�����������ͼ����ʾװ�����������飬ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ʵ������Ҫ������������ ��
��5��ͨ��������ʵ����̵ķ�������ʵ�����ʱ��Ҫ����_________������Ӧ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5mL 5% H2O2��Һ��������2�ε�Ũ�� FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݣ� | �Թ�A�в��ٲ������ݣ��Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5mL 5% H2O2��Һ��5mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com