��2013?��������ģ����ͼ��ʾΪʵ�����г�����������Ʊ�������������ռ�������ʵ��IJ����������Ը�����ĿҪ��
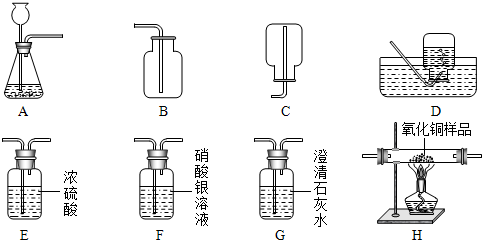
�ش��������⣺
��1����CaCO
3�����ϡ���ᷴӦ��ȡ���ռ���������Ķ�����̼��
����ѡ����������˳��Ϊ
AFEB
AFEB
����д���������ĸ����
�����ɶ�����̼ʱ��������Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
��
��2��С�ͬѧ����п��ϡ���ᷴӦ��ȡ�������ø���������ⶨij��������ͭ��Ʒ�Ĵ��ȣ�����Ϊ����ͭ����С�ͬѧ�����ʵ�鷽����ѡ���װ�ð�A��E
1��H��E
2��E
3˳�����ӣ�Ȼ�����ʵ�飨H�з�����Ӧ�Ļ�ѧ����ʽΪCuO+H
2��Cu+H
2O��E
1��E
2��E
3Ϊ3��ʢ��Ũ�����ϴ��ƿ�������Լ�����������վ���ȫ����
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
Zn+H2SO4=ZnSO4+H2��
Zn+H2SO4=ZnSO4+H2��
��
��װ��E
1��������
��ˮ��������
��ˮ��������
��
�۸�����ͭ��Ʒ����ǰ��С�ͬѧ����ʢ����ͭ��Ʒ�IJ�������ͨ����һ��ʱ�������������Ϊ���IJ����Ƿ���ȷ����˵������
��ȷ����ͨ������������װ���еĿ��������ⷢ����ը
��ȷ����ͨ������������װ���еĿ��������ⷢ����ը
��
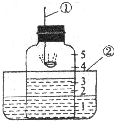
��С�ͨ��������Ӧǰ��E
2װ�õ������仯������������ͭ��Ʒ�Ĵ��ȣ����˷���������Ϊ������ͨ����������������ͭ��Ʒ���ȵķ�����
��������ͭ��Ʒ��Ӧǰ���������ͨ����ֵ���㴿��
��������ͭ��Ʒ��Ӧǰ���������ͨ����ֵ���㴿��
����Ӧһ��ʱ�����ֹͣ�Բ����ܵļ��Ȳ�����ͨ����������������ȴ��������Ʒȡ�������ֺ�ɫ�����д���������ɫ���壬��С褲�õ�����ͭ��Ʒ������ʵ��ֵ�ȽϽ�
ƫС
ƫС
���ƫ����ƫС����������һ�¡�֮һ����

 ��ͼ��ʾ���ô�װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�
��ͼ��ʾ���ô�װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�




 ��ͼ��ʾ���ô���װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�
��ͼ��ʾ���ô���װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�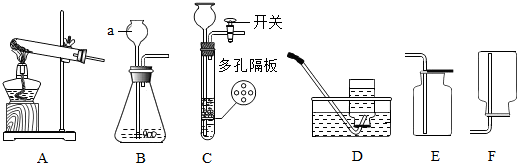
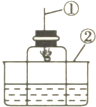 ��ͼ��ʾ���ô���װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�
��ͼ��ʾ���ô���װ�ò��������������ĺ�������ȼ�ճ��м�����ף�����������е�����������ѧ��Ӧ���������������壬�밴Ҫ����գ�